metals
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
metals
Metals
Solids at room temperature (except mercury)
Metals are made of a giant metallic lattice, where positive metal cations are surrounded by a "sea" of delocalised electrons.
The metallic bonds (electrostatic attraction between cations and delocalised electrons) are very strong, requiring high energy to overcome → metals are solids with high melting/boiling points.
Mercury is liquid at room temperature because relativistic effects weaken Hg–Hg bonding, lowering its melting point.
Shiny / lustrous appearance
The delocalised electrons in metals absorb and re-emit photons at the surface, causing reflection of visible light → metallic lustre.
Good conductors of heat and electricity
The mobile delocalised electrons can move freely throughout the lattice.
For electricity: electrons drift in response to an electric field, carrying current.
For heat: electrons transfer kinetic energy efficiently between atoms.
Malleable and ductile
Metallic bonds are non-directional (not fixed between specific atoms).
When force is applied, layers of cations slide over each other, and the delocalised electrons re-distribute → lattice doesn’t shatter but bends or stretches.
Non-Metals
Can be solids, liquids, or gases at room temperature
Non-metals form either:
Simple covalent molecules (e.g. O₂, N₂, Cl₂) held together by weak intermolecular forces → often gases or volatile liquids.
Covalent networks (e.g. diamond, silicon) → solids with very high melting points.
Their bonding explains why they don’t all share the same state at room temperature.
Dull and non-lustrous
Non-metals lack free electrons.
Covalent bonds/localised electrons cannot reflect light in the same way as metals → dull appearance.
Poor conductors of heat and electricity
In covalent or molecular structures, electrons are localised in bonds.
No delocalised electrons or free-moving ions → non-metals are insulators (exceptions exist like graphite, which conducts due to delocalised π electrons).
Neither malleable nor ductile (brittle instead)
Covalent bonds are directional (locked between specific atoms).
When stress is applied, these bonds break rather than allowing layers to slide → solids like sulfur or phosphorus are brittle and shatter easily.
semi metals
look like metals behave like non metals
are solid at room temperature
brittle and hard and not malleable generally
intermediate to fairly strong electrical conductivity
risk assessment template

isotopes
1. Position in the Periodic Table
Isotopes of an element have the same atomic number (Z) → they are the same element, so they occupy the same position in the periodic table.
Example: Carbon-12 and Carbon-14 both sit under C (Z = 6) in Group 14, Period 2.
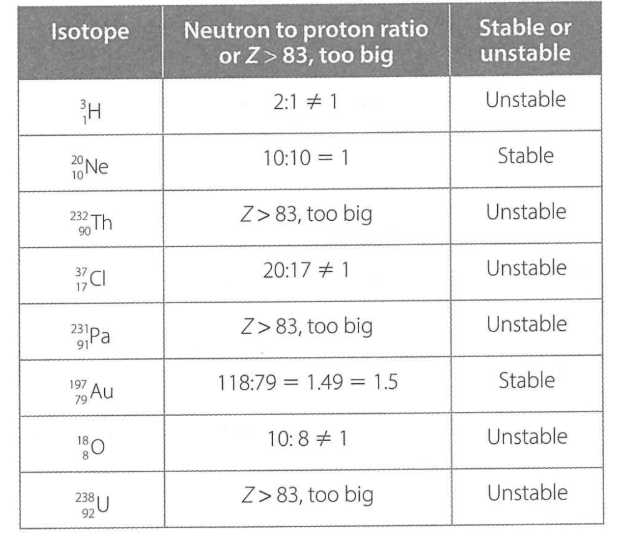
after Bi, everything in the zone of instability —🔹 Why elements after 83 are unstable
The nucleus is held together by the strong nuclear force (which acts between protons and neutrons).
Protons also experience electrostatic repulsion because they all have positive charge.
In lighter elements, having roughly equal numbers of protons and neutrons balances these forces, so the nucleus is stable.
In heavier elements (large atomic numbers), the number of protons is very high, so the repulsive force between them increases dramatically.
Neutrons can help stabilise by adding extra strong nuclear force without adding repulsion — but beyond Z = 83, no number of neutrons is enough to overcome the repulsion.
As a result, all isotopes with Z > 83 are unstable and will eventually decay (radioisotopes) by emitting α, β, or γ radiation to achieve greater stability)
standard atomic notation
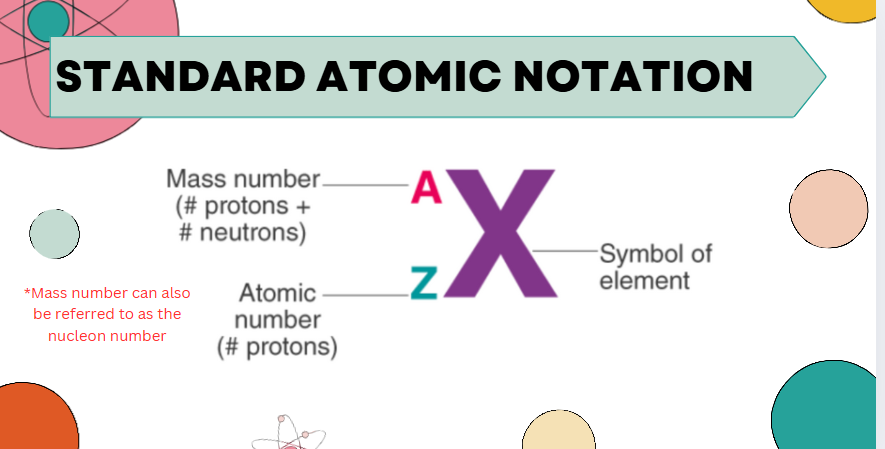
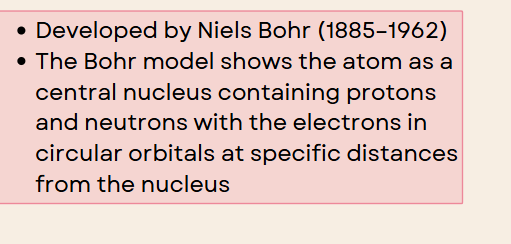
niel bohr
the bohr model shows an atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus
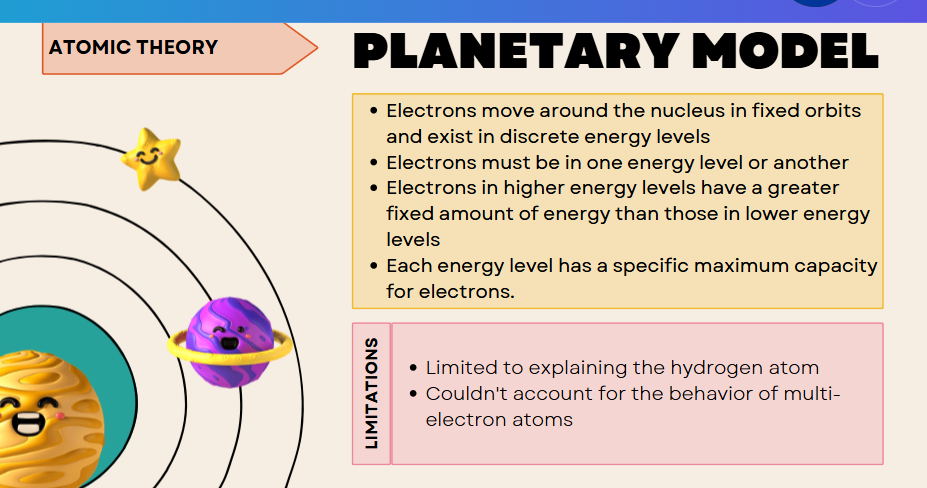
bohr model (focus + limitations)
fails because it cannot handle electron–electron interactions or orbital complexities.
compare and contrast the bohr and Schrodinger model
Sample Response:
Bohr’s Model described electrons moving in fixed circular orbits around the nucleus at discrete energy levels. It explained hydrogen well but failed for multi-electron atoms.
Schrödinger’s Model treated electrons as matter waves and used the Schrödinger equation to calculate the probability of finding electrons in orbitals. Electrons exist in 3D regions (s, p, d, f orbitals) rather than fixed paths, making this model successful for multi-electron atoms.
In summary: Bohr = fixed orbits; Schrödinger = orbitals and probabilities.
describe the distribution of the atom
Question: Describe the distribution of mass and charge within the atom.
Sample HSC Response:
The atom consists of a dense, central nucleus that contains almost all of the atom’s mass and is positively charged. Within the nucleus are protons, which are small positively charged particles, and neutrons, which are neutral particles with a slightly greater mass than protons. Surrounding the nucleus are electrons, which are tiny negatively charged particles with a mass of about 1/2000th of a proton. Electrons occupy regions of space called the electron cloud, which accounts for the majority of the atom’s volume, but very little of its mass.
Thus, the mass of the atom is concentrated in the nucleus, while the electrons are distributed throughout the electron cloud, giving the atom its overall volume and balancing the positive charge of the nucleus.
ion notation
instability
electrostatic forces- repulsion among positively charged protons
nuclear force- attraction between protons and neutrons
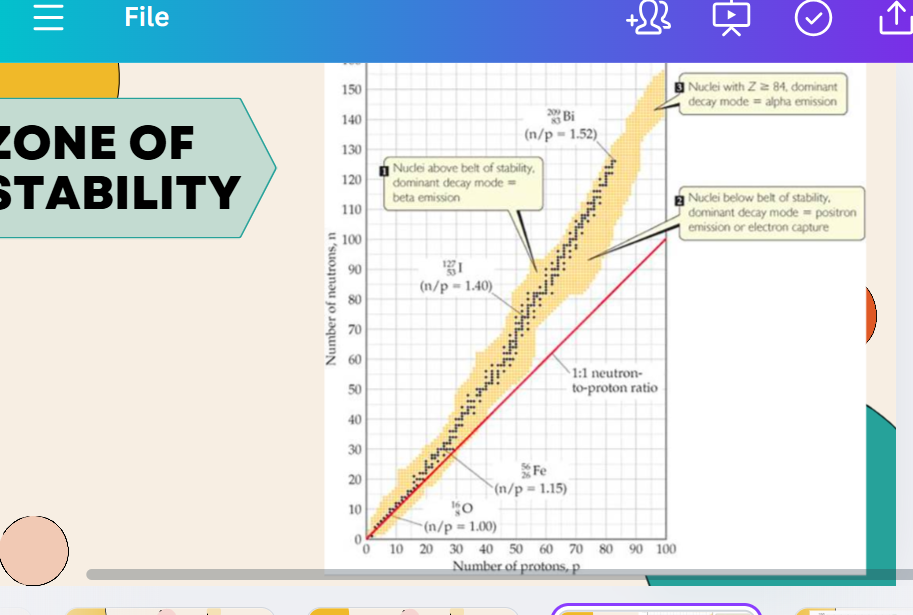
in larger nuclei (larger than 83), electrostatic forces overpower short range attractions, causing instability
stable nuclei with atomic numbers up to 20 need 1:1
stable nuclei with atomic numbers between 20 and 83 need to have a neutron: proton ratio of about 1.5:1
ZONE OF STABILITY
if n:p ratio is outside this range, then the isotope is unstable
if the ratio is too high, the isotope is a beta emitter, if it is too low the isotope is an alpha emitter
types of radiation
alpha
beta
gama
alpha
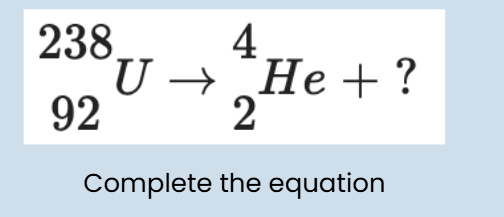
beta particle
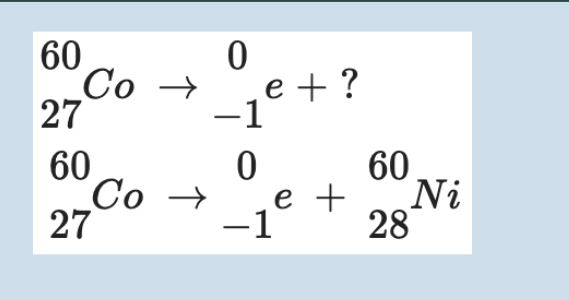
gama

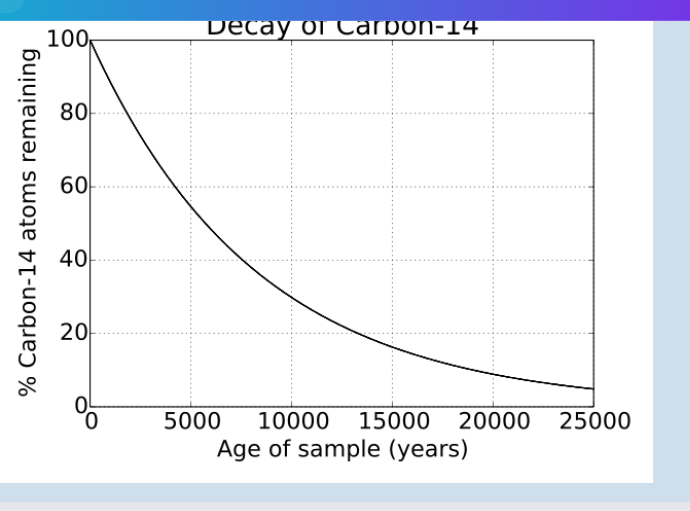
half life
time taken for half of a given sample of radioisotopes to undergo radioactive decay
example: half lie: 5730 years—if yu started with 1kg of carbon-14, after 5730 years only 0.5kg of the carbon 14 will be left (the half will have undergone radioactive decay)
Tennessee
after element number 83, hence larger nuclei—too many protons—the electrostatic repulsion is too strong—no neutrons can counterbalance this—-emitting radiation—-beta emission
* unstable isotopes undergo the process of radioactive decay—as time passes, less of the radioactive isotope will be present—the half life of a radioisotope is the time needed for half the atoms in a given sample to undergo radioactive decay
human made radioisotopes —technetium099m
used in med for diagnostic imaging of the brain, kidneys, bone, etc
the isotope is administered to the specific area and the gamma radiation is detected and an image can be produced of what’s happening inside the body
short half life of around 6hrs and rapid excretion from the body limits its toxic effects
th99mTc decays to 99Tc via the release of gamma rays and low enrgy electrons—gamma rays emitted—-this is detected by a gamma camera
add info
Investigating the Properties of Unstable Isotopes
Unstable isotopes, also known as radioisotopes, have nuclei with an imbalance of protons and neutrons, causing them to spontaneously decay and emit radiation. This radiation can take the form of alpha (α), beta (β), or gamma (γ) radiation, each with distinct properties.
Alpha radiation (α): Consists of 2 protons and 2 neutrons (helium nucleus). It has very high ionising power but very low penetrating power, stopped by paper or skin. Alpha emitters (e.g. radon) can cause significant internal damage if inhaled or ingested.
Beta radiation (β): Consists of high-energy electrons (β⁻) or positrons (β⁺) emitted when a neutron converts into a proton or vice versa. It has intermediate penetrating power (stopped by aluminium) and moderate ionising power. For example, iodine-131 undergoes β⁻ decay and is used medically to destroy thyroid tissue.
Gamma radiation (γ): High-energy electromagnetic photons with no mass or charge. Gamma rays have very high penetrating power (requiring lead or concrete shielding) but low ionising power. Gamma-emitting isotopes such as technetium-99m are used for diagnostic imaging as the radiation can leave the body and be detected by scanners with minimal cellular damage.
These properties explain why different unstable isotopes are suited to different applications in medicine and industry. For instance, isotopes that emit gamma rays (like Tc-99m or I-123) are ideal for diagnostic imaging, whereas isotopes that emit beta radiation (like I-131) are used for therapy, as their stronger ionising radiation destroys diseased tissue.
stable or not
periodic table
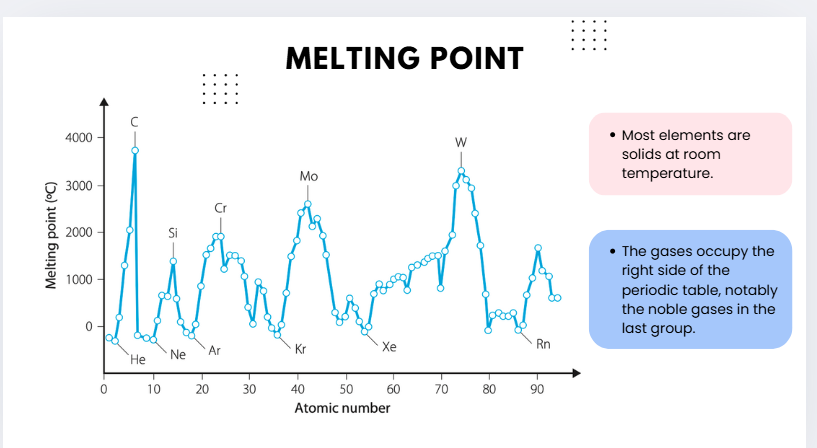
melting point graph questions
Sample Response:
The melting point of elements shows clear periodic trends across the periodic table due to differences in bonding and structure. In Groups 14 elements such as carbon and silicon, melting points are extremely high because they form giant covalent network structures with strong covalent bonds throughout the lattice. Transition metals such as chromium, molybdenum and tungsten also have very high melting points due to strong metallic bonding involving delocalised electrons and closely packed positive ions. In contrast, the noble gases such as helium and neon have extremely low melting points as they exist as monoatomic gases held together only by weak dispersion forces. Similarly, molecular non-metals such as oxygen and nitrogen have low melting points due to weak intermolecular forces.
Across a period, melting points generally increase in the metals, peak at the network covalent elements (e.g., C, Si), and then decrease sharply in the non-metals and noble gases. Down a group, trends vary: melting points decrease in the alkali metals, remain high but irregular in the transition metals, and increase slightly in the noble gases due to stronger dispersion forces with greater atomic mass.
Thus, the melting point trend reflects the type and strength of bonding present in the element, with giant covalent and metallic structures producing high melting points, while weakly bonded molecular and noble gas elements produce low melting points.
***Transition metals have high melting and boiling points due to strong metallic bonding from delocalised d and s electrons. The trend peaks in the middle of the block (e.g. W, Mo, Cr), but does not increase simply with mass — instead it depends on the number of delocalised electrons and bond strength.
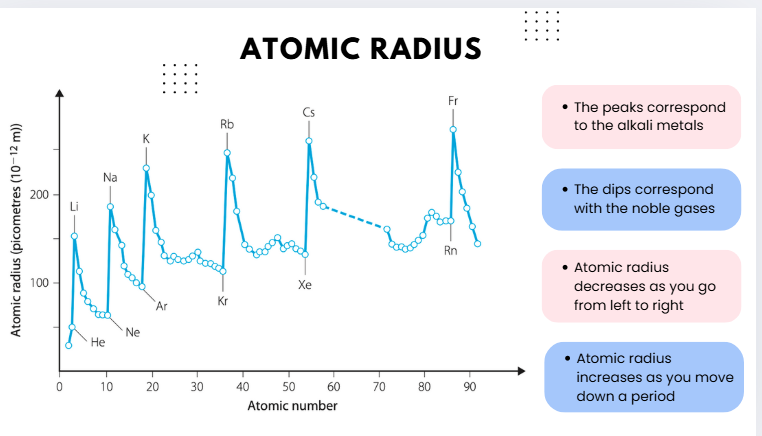
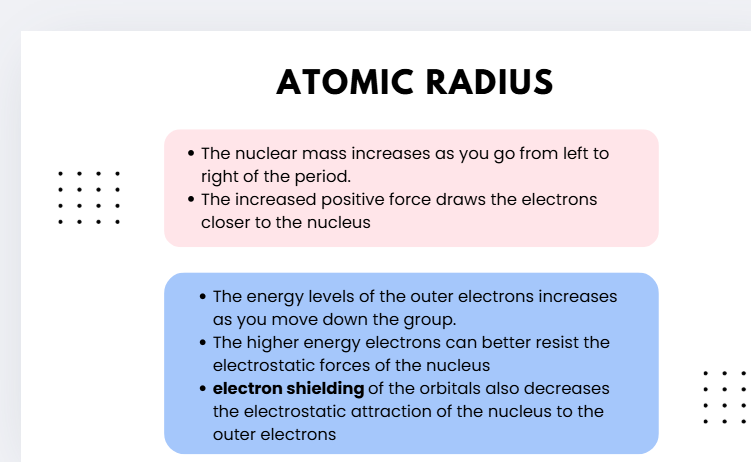
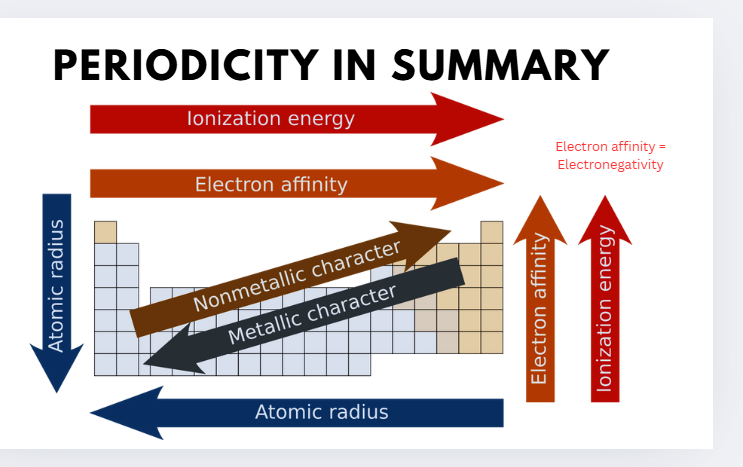
atomic radius
the distance from an atom’s nucleus to the outermost orbital of electron
AR graph
2nd AR diagram
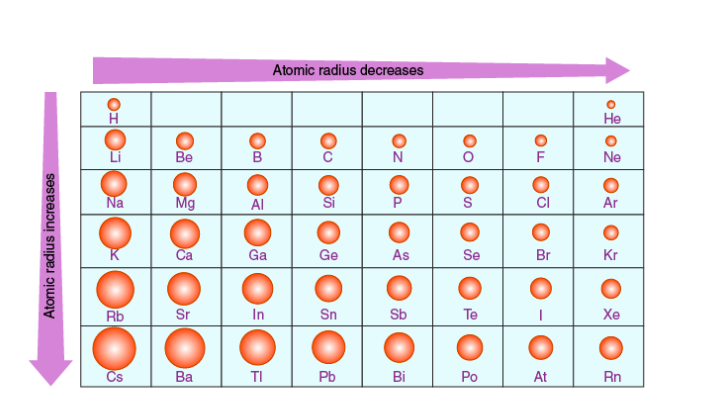
AR explanation
🔹 Across a Period (left → right)
Nuclear charge increases (more protons).
Electrons are added to the same shell, so shielding doesn’t really increase much.
The stronger positive attraction pulls electrons closer to the nucleus.
🔽 Atomic radius decreases across a period.
Example HSC response:
Across Period 2, the atomic radius decreases from lithium to fluorine because the number of protons increases, pulling electrons closer to the nucleus without significant additional shielding.
🔹 Down a Group (top → bottom)
More electron shells are added (higher energy levels).
Increased electron shielding reduces the pull of the nucleus on outer electrons.
Outer electrons are therefore further from the nucleus.
🔼 Atomic radius increases down a group.
Example HSC response:
Down Group 1, atomic radius increases from lithium to cesium because additional electron shells and shielding outweigh the increasing nuclear charge, so outer electrons are held less tightly.
ionisation energy meaning
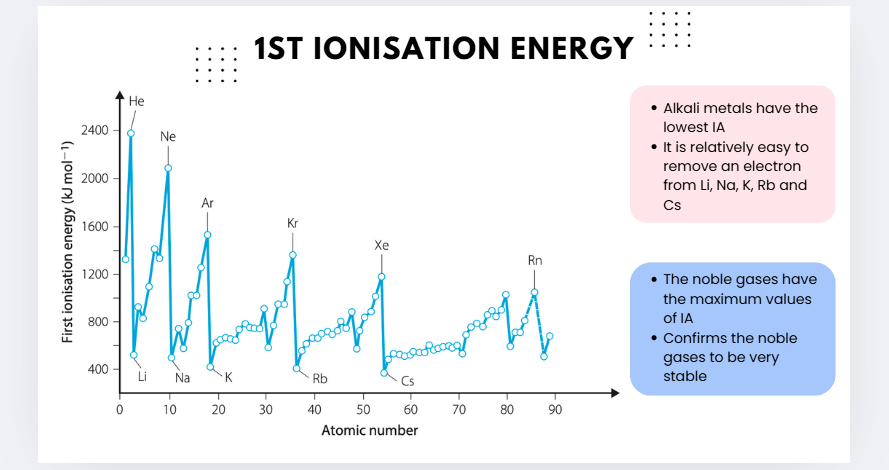
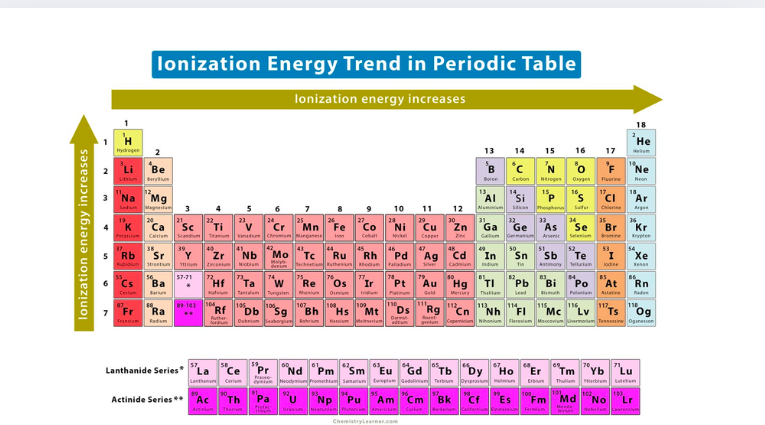
IONISATION ENERGY
First ionisation energy (IA): the energy required to remove an electron from a neutral gaseous atom of the element
Lower the ionisation energy: easier to remove an electron
Each element has several ionisation energies, called the first, second, third (and so on) ionisation energies.
The second ionisation energy is higher than the first because removing an electron from a positive species requires more energy than removing it from a neutral species.
first ionisation (graph)
periodic table graph
1st ion explanation

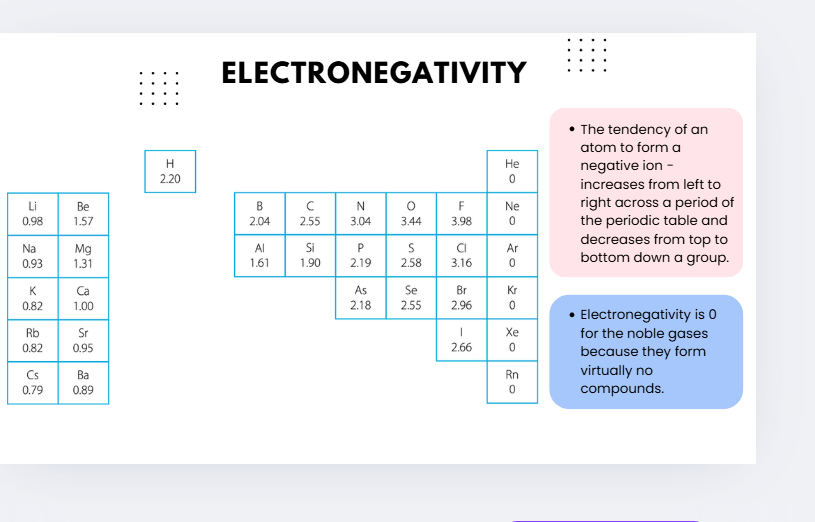
electronegativity trend 1
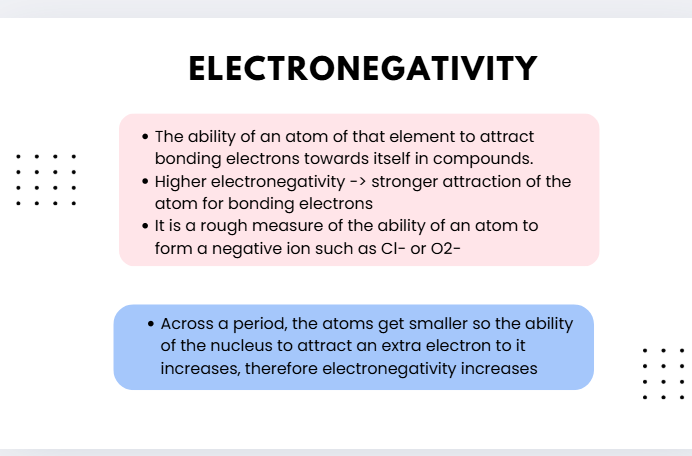
electronegativitu trend 2
reactivity with water (trend)

periodicity in summary





explain the trends in electronegativity on the periodic table

order the following elements by increasing electronegativity : su,fur, oxygen, neon, aluminium
EFFECT WOUDL CONTRIBBUTE MORE***

four elements of atomic numbers 17, 18, 19 and 20 have the first ionization energies of 1500, 600, 400 and 1300 Kj mol-1 , which first ionization energy belongs to which element? explain how you reached your decision?


last question
nuclear charge, shielding effect. AR—maximise the attraction——number of shells + number of protons

question

question 1

question 2

answer

question

question

question
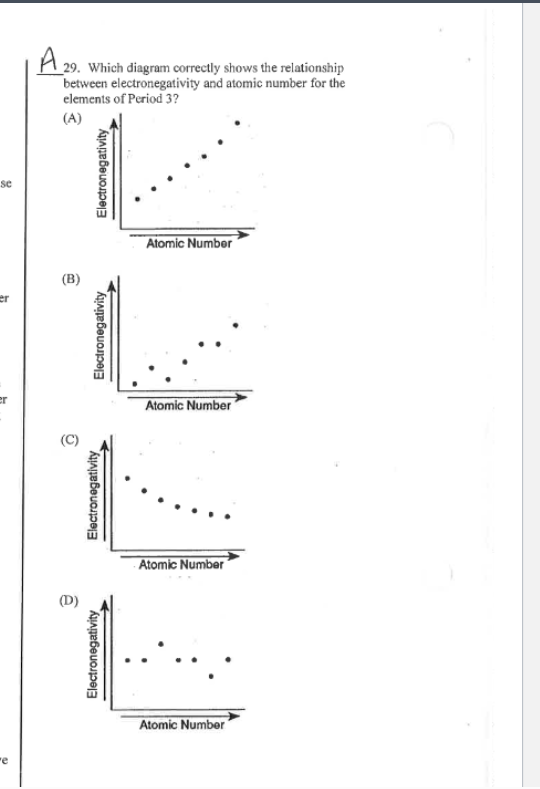
electronegativity notes
bonds: covalent, ionic and metallic
noble gases have a complete outer shell of valence electrons (stable and unreactive)
other atoms can obtain noble gas configuration by transferring or sharing electrons **
metallic bonding
in metals
the metal ions are surrounded by a sea of shared electrons
the strong electrostatic attraction between those oppositely charged particles
the strong giant lattice structure maximizes the attractive forces
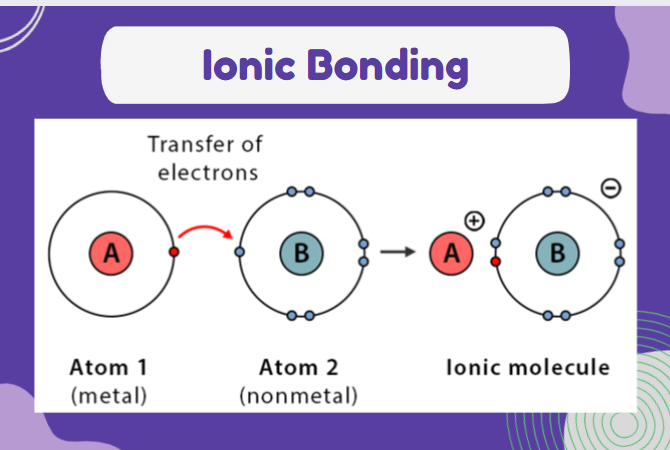
ionic bonding
between a metal and non metal atom
electron transferred from one atom to another in order to get a noble gas configuration
the strong electrostatic attraction holds the ions together in an ionic bond
covalent bonding
a chemical bond formed between two atoms as a result of sharing a pair of electrons
the electrostatic force the nuclei experience towards the electrons holds the molecule together
electronegativity
the ability of an atom of that element to attract bonding electrons towards itself in compounds
higher electronegativity— stronger attraction of the atom for bonding electrons
forming a covalent compound
if the atoms have similar attractiosn for wlectrons, they are more likely to form a covalnt compound (if different smaller than 1.5)
forming an ionic compound
one atom must have strong attraction for electrons than the other
(if the difference is greater than 1.5)