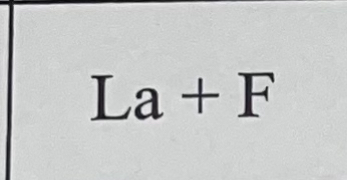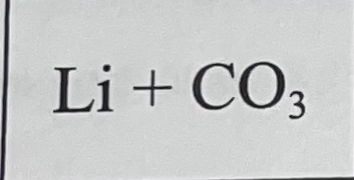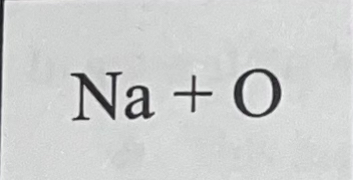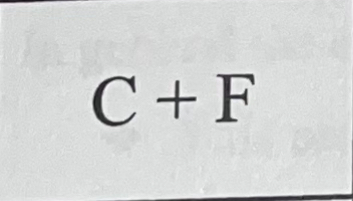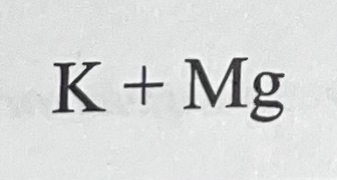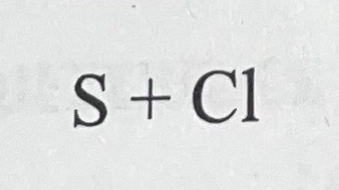covalent bonds quiz review
1/24
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Metal plus nonmetal makes…
Ionic bond
Nonmetal plus nonmetal makes…
Covalent bond
Noble gas plus metal makes…
Doesn’t make a bond
Metal plus metal makes…
Metallic
What molecule/prefix contains one atom?
Mono
What prefix/molecule contains 2 atoms?
Di
What molecule/prefix contains 3 atoms
Tri
What molecule/prefix contains 4 atoms?
Tetra
What molecule/prefix contains 5 atoms?
Penta
What molecule/prefix contains 6 atoms?
Hexa
What molecule/prefix contains 7 atoms?
Hepta
What molecule/prefix contains 8 atoms?
Vota
What molecule/prefix contains 9 atoms?
Nona
What molecule/prefix contains 10 atoms?
Deca
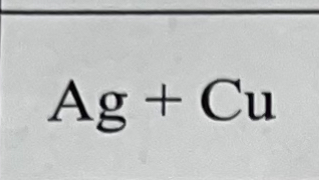
What bond do they make when combined, and why?
They make a metallic bond because they’re both metals.
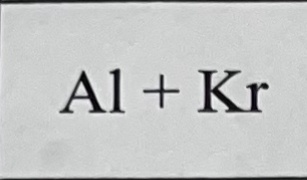
What bond do they make combined and why?
They don’t make a bond because Kr is a noble gas.
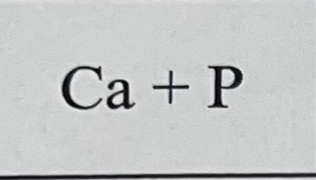
What bond do they make combined and why?
They make an ionic bond because Ca is a metal and P is a non metal.
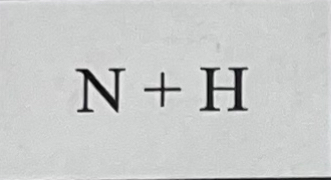
What bond do they make combined and why?
They make a covalent bond because they’re both non metals.