Science-Classifying Materials-Junior Cycle
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Matter is split into………..
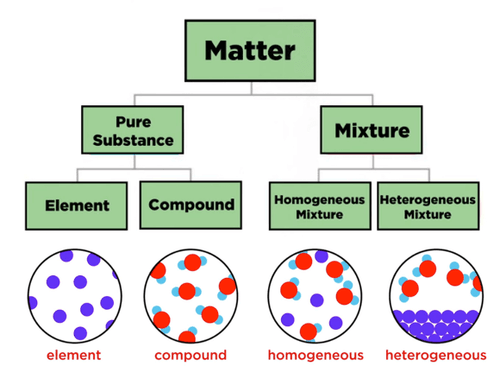
Pure Substance
Made up of particles that are exactly the same sa each other
Mixture
Made up of tow or more different substances mingled together but are not chemically combined. An object will float if its density is less than the medium
Element
Pure substance which cannot, by chemical means be broken down onto simpler substances
>Smaller number=Atomic number
>Atomic number=number of protons
Compounds
Pure substance made from two or more elements which are chemically combined. Completely new substance with its own properties
Molecules
Smallest part of an element that can exist on it’s own. Elements that make up compounds, always present in definite fixed amounts.
Physical Change
No new substance formed
Chemical change
New substance created
Properties of metals
>Lustrous
>Solid, except for Hg
>Malleable
>Ductile
>Sonorous
>Dense and strong
>High melting points
>Good conductor of heat and electricity
>Most metals corrode
Corrosion of metals
When they react with oxygen in the air. Oxidization, Magnesium + Oxygen = Magnesium oxide
Properties of non metals
>Poor conductor of heat
>Poor conductor of electricity
>Dull
>Have low boiling point