(1.18-1.24) The Periodic Table
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
How are elements arranged on the periodic table?
In order of atomic number
In groups and periods
What is a group and what does it represent?
The columns and the number of electrons in its outer shell.
What is a period and what does it represent?
The rows and the number of shells.
How can the electrical configuration of the first 20 elements be deduced from the Periodic Table?
Shells hold electrons in the formation [2,8,8] so that can be used with the atomic number- which is the number of electrons.
Why do elements in the same group have similar chemical properties?
If elements have the same number of electrons in the outermost shell, they share properties.
How does electrical conductivity help classify elements?
Metals and conductive, non-metals are not
How does the acid-base character of oxides help classify elements?
Metals have basic oxides, giving salt and water when reacting with acids.
Non-metals have oxides which are acidic or neutral.
Where is the separation between the metals and non-metals on the periodic table?
The elements in the right corner + hydrogen are non-metals.
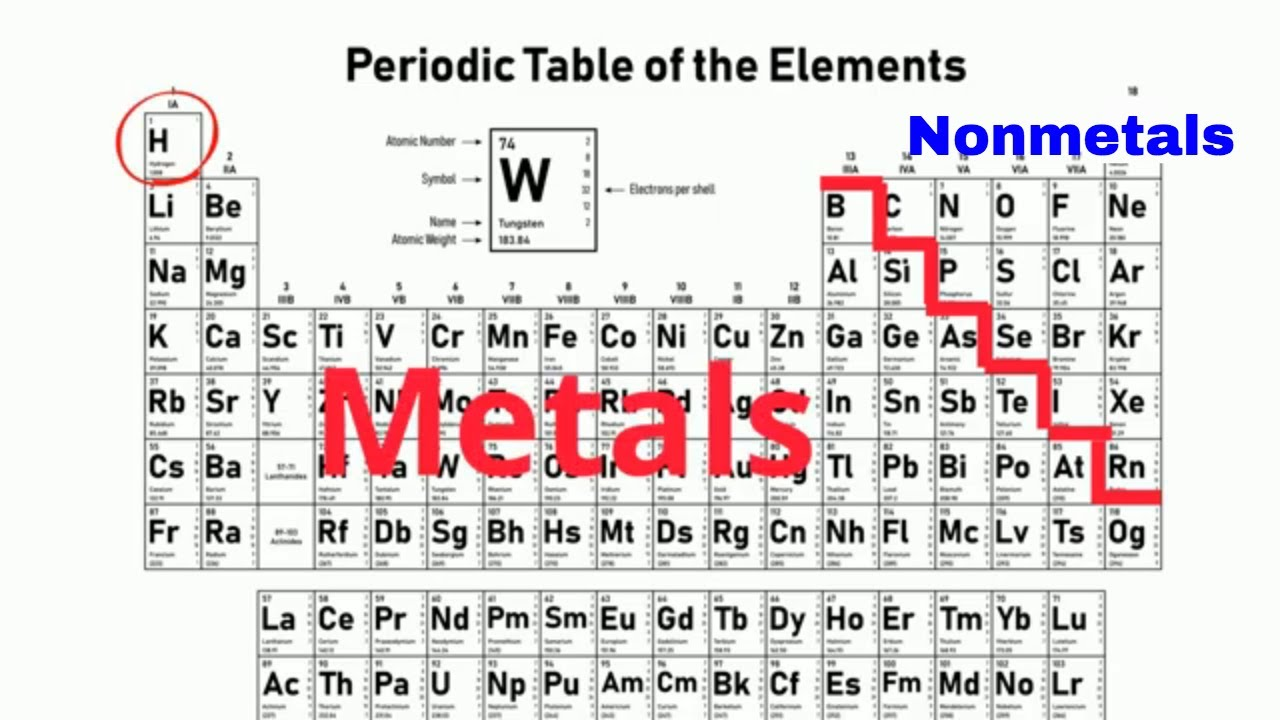
Why do noble gases (Group 0) not readily react?
They have a full outer shell of electrons so they are inert.