ALL TOPICS FOR EXAM 1
1/145
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
146 Terms
What are the most common bulk elements?
CHNOPS (Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur) are the most common bulk elements essential for life.
Others: Na, K, Ca, Cl
What are the 2 type of elements we have in biochem?
bulk elements (most common)
trace elements (needed in small amounts)
What are the trace elements?
Iron (Fe), Zinc (Zn), Copper (Cu)
Give an example of when a trace element was toxic in large amounts…
iron dose in kids with Flintstones vitamins.
Difference between single bonds and triple bonds?
single bonds - longer, weakest
double bonds- shorter, strongest
What is the difference between isomers and stereoisomers
isomers
same chemical formula, but different structure/arrangement (could be bonds or 3D)
stereoisomers
same chemical formula and same bonds, but only 3D arrangement is different.
they are chiral molecules that form stereoisomers
special type of isomers
what are the 2 types of isomers?
configuration- breaking bonds
confirmation- not breaking bonds. shape changes via bond rotation.
What is stereochemistry?
The study of 3D shapes of molecules
What is a chiral carbon?
a carbon attached to four different group
What makes a molecule chiral?
a chiral carbon
What is main difference between chiral/achiral molecules AND stereoisomers?
Chiral/Achiral molecules are about 1 molecule itself, and stereoisomers are compared to 2 molecules
What is difference between chiral and achiral
chiral - “handedness” (mirror images cannot be lined up - not superimposable)
achiral - mirror images can line up perfectly - superimposable
What are the 2 types of stereoisomers?
enantiomers - mirror image stereoisomers (like right and left hand) and not superimposable
diastereomers - stereoisomers are not mirror images (same formula, 3D arrangement is different)
What type of molecules can form stereoisomers?
choral molecules only!
Tell me about a case that shows the importance of stereochemistry?
Case: Thalidomide
Used in 1950s-60s for morning sickness (Europe)
One enantiomer = effective against morning sickness
Other enantiomer, called teratogenic= caused birth defects (phocomelia)
Phoco means seal in spanish
Drug was racemic (mix of both enantiomers)
Later discovered even pure enantiomer converts in body
Even when chemists later made only the good enantiomer (called enantiomer-selective synthesis), something tricky happened:
in the human body (in vivo), the good enantiomer converted into the bad one.
So even if you give just the safe version → your body ends up with both.
Still used with extreme caution (must avoid pregnancy)
Is Thalidomide still prescribed?
It is now used very carefully for certain diseases
What are the restrictions to use Thalidone?
If you are someone who can get pregnant, you must promise (legally) to:
Not become pregnant.
Use 2 types of birth control if sexually active.
What is the modern example of Thalidone?
Zofran (ondansetron)
Used for nausea/vomiting (pregnancy, chemo)
Orally dissolving tablet (ODT)= fast-acting
Originally cost $100/tablet, now cheaper
Why Stereochemistry Matters in Medicine?
Stereochemistry matters because it affects how well a molecule (drug) can fit into a protein’s (enzymes) binding site.
Enzymes can tell the difference between two mirror image versions (stereoisomers) of a molecule — and they may only work with one of them.
So, the shape of the drug determines whether it works or not.
What are the 4 classes of biomolecules?
Proteins
Carbohydrates
Nucleic Acids
Lipids
What are each of the 4 classes of biomolecules made from?
Proteins
made of chains of amino acids (20 or 21) folded into 3D structures
Nucleic Acids
made of nucleotides
Lipids
made of fatty acids
Carbohydrates
made of monosaccharides
What are the functions of each of the 4 classes of biomolecules?
Proteins
catalysts (enzymes)
protein catalysts enhance the rate of chemical reactions
transporters (hemoglobin)
Nucleic Acids
DNA: store and transmit genetic information
ATP: used for energy transfer
Lipids
long-term energy storage: triglycerides (fats)
neutral lipids (no charge)
hydrophobic
membrane structure: phospholipids (form bilayer)
amphipathic
Carbohydrates
short/medium energy storage:
glycogen (animals)
starch (plants)
information
cell surface decorating
immune recognition
blood groups
signaling: communications between cells.
What is the structure of each of the 4 classes of biomolecules?
Proteins
Central carbon (C)
Amino group (–NH₃⁺)
Carboxylic acid group (–COO⁻)
Hydrogen (H)
R group (variable) → determines properties (hydrophobic/hydrophilic, acidic/basic)
Nucleic Acids
Nitrogenous base (A, T, G, C, U)
Sugar (ribose in RNA, deoxyribose in DNA)
Phosphate group(s)
Lipids
Triglycerides: Glycerol + 3 fatty acids
Phospholipids: Glycerol + 2 fatty acids + polar head group
Hydrophilic (polar) head + hydrophobic (nonpolar) tails
Amphipathic → forms membranes
Carbohydrates
Made of sugars (mono-, di-, polysaccharides)
Monosaccharide = glucose
Polysaccharides = glycogen
Polysaccharides can be branched
More branching = faster breakdown (enzymes act at many ends)
Less osmotic pressure than single glucose molecules (prevents cell rupture)
What are the differences between the DNA and ATP structure?
Key Difference:
DNA nucleotides = 1 phosphate → info storage
Double stranded - double helix
Deoxyribose backbone
Has T base
ATP = 3 phosphates → energy
Single stranded
Ribose backbone
Has A base
What are the levels of proteins?
Primary: Amino acid sequence (linear chain) of polypeptide
Secondary: Alpha helices & beta sheets (H-bonding)
Tertiary: Full 3D shape of one secondary chain
Quaternary: Multiple chains together into a functional froup (e.g., hemoglobin)
How does the body prefer to use energy? Give me first to last…
Glucose used first
When glucose runs out → glycogen is used
When glycogen is gone → body uses fats (fatty acids sent to mitochondria)
What are the central principles of life?
Cells = fundamental unit of life.
Use a small set of carbon-based molecules to:
Build complex structures
Store genetic info (DNA/RNA)
Life = dynamic steady state, not equilibrium
Dead = equilibrium
Cells can self-replicate and self-assemble.
Life evolves over time.
Cytosol
Fluid inside the cell but outside the organelles
oxidative environment (takes electrons, “electron thief room”).
Mitochondrial matrix
Inside the mitochondria
reducing environment (gives electrons, “electron donor room”).
Site of fuel oxidation
Lysosome
Acidic (low pH).
Garbage/recycling center of the cell (contains digestive enzymes)
If it bursts → enzymes spill out but stop working (pH not acidic outside) → protects the cell.
At what specific pH does every enzyme work best?
every enzyme has an optimal pH where it woks best
Same enzymes can work in _________ depending on compartments.
opposite directions
Where does drug metabolism happen?
Smooth ER
Rough ER
ribosomes attached → makes proteins that will be exported or put in membranes.
Smooth ER
no ribosomes → makes lipids (fats, steroids, membranes) + breaks down drugs (drug metabolism).
Have single membrane
Free ribosomes
floating in cytosol → make proteins that stay in the cytosol.
ER
Abundant form of cytoplasmic membrane
Cytoplasm
Not just fluid!
Contains the cytoskeleton (scaffolding):
Gives cell shape.
Helps with movement inside the cell
Organelle
A membrane-enclosed structure within a eukaryotic cell
who have a single membrane
who have double membrane
ER and Golgi Complex
Nucleus, Mitochondria, Chloroplast
What are bilayer membranes made of?
phospholipids
Hydrophilic heads = face water (outside & inside).
Hydrophobic tails = face inward, away from water
What do bilayer membranes act as?
Acts as a barrier between inside and outside of cell.
How do different molecules cross bilayer membrane?
Nonpolar molecules (hydrophobic) = pass easily (“like dissolves like”).
Polar molecules (hydrophilic) = blocked by hydrophobic interior → need transporters/channels.
How does nuclear membrane diff from other membranes?
It is perforated with pores to facilitate transport between the nucleus and cytoplasm
Tell me whole process of from how a protein is made and shipped out of the cell
TRANSCRIPTION: mRNA is made from 1 DNA strand formed in the nucleus
TRANSLATION: mRNA goes to rough ER, ribosomes make Protein
Transport vesicles: protein is packed into vesicles and delivered to the Golgi complex
Short & Pack Protein in Golgi Complex
Shipping Vesicles carry out protein from the Golgi
Exocytosis: vesicles fuse with the cell membrane and the protein exits the cell
Most cells have same WHAT?
DNA
What is difference between Eukaryotic and Prokaryotic?
Eukaryotic
Have nucleus
Found in plants and animals
Contain membrane-bond organelles
Prokaryotic
Have no nucleus
Found in bacteria
What is DNA replication?
recopy (DNA to DNA)
What is DNA replication final result?
semiconservative (each new DNA has 1 old strand and 1 new strand)
How does DNA replication occur?
requires a DNA polymerase
duplicates both DNA strands
bidirectional - replication starts at an origin and goes both ways
What determines the fate of a cell?
Selective Expression = depends on:
SPATIAL-place (which genes are transcribed)
TEMPORAL- time (when)
Alternative splicing
1 gene → multiple proteins
Reverse transcription
RNA → DNA (used by viruses).
nucleus
contains the genes (chromatin)
plasma membrane
separate the inside of the cell from the outside
endosome
carries important biochemicals into cell
chloroplast
site of photosynthesis
secretory granules
destined for fusion with the plasma membrane
What are the 2 forms that amino acids can exist in?
L isomer: amine group (NH₃⁺) on the left in Fischer projection.
D isomer: amine group on the right.
These are enantiomers (non-superimposable mirror images).
Where are each form of amino acids used?
Life overwhelmingly uses L-amino acids in proteins.
D-amino acids are rare in nature.
Exception: Some bacteria have D-amino acids in their cell walls.
This acts as a defense mechanism, because human enzymes are built to recognize and break down L-amino acids, not D.
→ Professor flagged this as an exam question.
what are different representations of amino acids?
Ball-and-stick model (3D).
Simplified stick model (CH₃, COO⁻, NH₃⁺, H).
Fischer projections → flat 2D version used in biochemistry to quickly show stereochemistry.
Which one amino acid R groups that is from the polar, uncharged area is different?
cysteine (Cys - C)
When deprotonated, it becomes a negative charge
When protonated, it becomes neutral and has pK of 8
It's different because those polar, uncharged side chains are always neutral at pH=7
tell me the R group that turns amino acid into non chiral molecule?
glycine (Gly-G)
Tell me about the special R group found in the positively charged area
Histidine (His-H)
pKa ~6 → borderline at physiology.
can be neutral (drop of H) or positive (pick up H).
Enzymes exploit this — e.g., in carbonic anhydrase, the surrounding protein environment shifts histidine’s pKa closer to 7.4, making it exactly balanced to act as a proton shuttle.
How many amino acids are there?
Beyond the “big 20,” there are 250–400 amino acids that show up in proteins.
What are the common PTM (post-translation modification) he mentioned…
hydroxyproline
Cysteine modifications- disulfide bond
selenocysteine (NOT PTM!!!)
What is hydroxyproline?
a modified version of proline (Regular proline has no OH groups, but enzymes can modify it to add one)
This OH allows hydrogen bonding → forms collagen’s triple helices and helps stabilize collagen.
Collagen = a key connective tissue protein.
Pharmacists often see patients (esp. older adults) asking about supplements for collagen repair.
How are disulfide bond (S–S) formed?
Two cysteine residues can be oxidized (loss of electrons and Hydrogen) to form a disulfide bond (S–S)- covalent bond
This can happen even if the cysteines are far apart in the primary sequence, as long as they’re close in 3D space.
Reduction = gain of electrons → bond breaks back into two –SH groups.
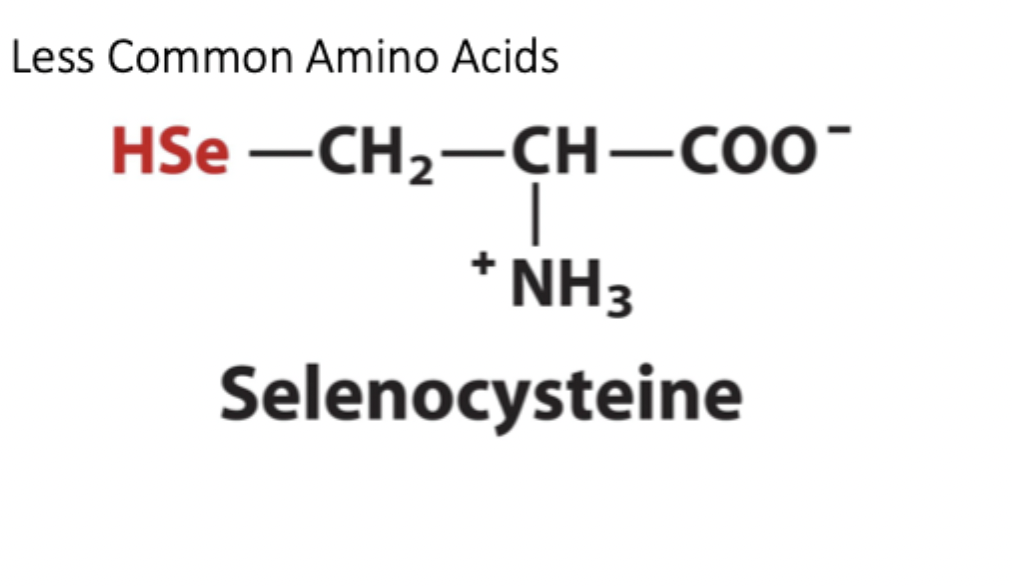
What is special about Selenocystein?
only amino acid that isn’t a post-translational modification. is the “21st amino acid,” unique because it hijacks translation directly.
Clarification:
For something like hydroxyproline: proline is incorporated first, then later modified by an enzyme.
For selenocysteine: it is already in its final form before insertion into the peptide.
incorporated during translation directly into the growing polypeptide chain.
Achieves this by “stealing” another amino acid’s tRNA.
All other “extra” amino acids (like hydroxyproline) come from modifications made after the protein is assembled.
What is PI?
How to find PI?
PI- Isoelectric Point - the pH at which the molecule’s overall net charge = 0.
Find the PI by taking the average of the pKa values
How does selenocysteine look?
What it titration curve?
In theory, you can use the titration curve shape to guess the amino acid:
He called this “dumb in practice” (not super reliable), but he wanted you aware of it.
He promised: you won’t have to identify amino acids from titration curves on the exam.
What are the main patterns to know about a titration curve?
Two clean steps → simple amino acid, no ionizable side chain (like glycine, alanine, valine).
Extra bumps tell you if there’s an acidic or basic side chain.
Extra bump in acidic region (~pH 4) → acidic side chain (Asp, Glu).
Extra bump in basic region (~pH 10–12) → basic side chain (Lys, Arg, His).
Extra bump in the middle (~6) → histidine
another way to look at it- take 1 amino aicd and see how it changes in pH as you add base.
What does the titration graph show?
x axis - how much base (OH-) added
y-axis- pH of solution
the more base, the higher the pH (less acidic)
the lower the base, the lower the pH (more acidic)
What are essential amino acids?
Essential amino acids = ones your body(enzymes) cannot make.
You must eat them in your diet.
What are dietary challnegs of getting all esential amino acids?
Getting all essential amino acids can be difficult without meat.
Possible for vegans, but requires a variety of protein sources (beans, legumes, grains, etc.) so all essential AAs are covered.
explain to protonation and deprotonation of the carboxyl group and amine group.
Amine group (–NH₂ / –NH₃⁺):
pKa ≈ 9.6.
Below 9.6 → protonated (NH₃⁺), positive charge.
Above 9.6 → deprotonated (NH₂), neutral.
Carboxyl group (–COOH / –COO⁻):
pKa ≈ 2.3.
Below 2.3 → protonated (COOH), neutral. .
Above 2.3 → deprotonated (COO⁻), negative charge.
what is zwitterion
At physiological pH (~7.4):
Amine group is protonated (+).
Carboxyl group is deprotonated (–).
Net result = zwitterion (molecule with both + and – charges, but overall neutral).
Give me pK values of amine group and carboxly group.
amine- 9.6
carboxyl- 2.3
what does pK and pI mean?
pK values= the pH at which it is half protonated and deprotonated.
pI - the whole amino acid has no net charge
Why water important in biochem?
(human body) cells are ~70% water → chemistry happens in aqueous solution.
What keeps an aqueous solution stable?
osmotic pressure (push water makes when it tries to move into an area with more dissolved stuff) , noncovalent interactions (weak attractions between molecules, not strong bond), and buffering keep biology stable.
What features affect how solute behave in water?
Properties of Liquids
Volume is fairly constant, unlike gases.
Liquids take the shape of their container — just like cats!
In water, how do biomolecules stick/recognize each other?
via noncovalent (not covalent) forces:
ionic, hydrogen bonds, van der Waals, plus the hydrophobic effect (discussed later).
Tell me about all the non-covalent interactions:
1. Ionic Bonds (Electrostatics)
Governed by Coulomb’s law. Strength increases with higher charges and shorter distance.
Shielding Effects
Shielding/dielectric of water weakens long-range attraction/repulsion.
EXPLAIN IN DETAIL (FOR ME)
Water and other molecules can “shield” charges, reducing electrostatic interactions.
Without water: + and – stick strongly.
With water: attraction is weaker because water molecules surround them and block the pull.
2. Hydrogen Bonding
a weak “magnet-like” attraction between molecules.
A special dipole-dipole interaction.
Requires:
Hydrogen atom.
Two electronegative atoms (e.g., O, N, F).
One of these atoms must be bonded to H, the other must be nearby.
Partial charges attract each other.
Stronger than most dipole-dipole, but ~1/100th strength of covalent bonds.
Important Example: Holds DNA strands together (also protein structure, enzyme binding)
3. Van der Waals Interactions
Very weak, but important in large numbers.
Temporary induced dipoles in nonpolar atoms/molecules. Individually weak, add up over many contacts.
Example: Fats in steak solidify when cooled — due to many van der Waals interactions.
What’s the hydrophobic in water?
Non-polar molecules don’t mix with water.
Water molecules push them together, leading to:
Protein folding: Hydrophobic residues go inside; hydrophilic residues outside
Give real-life examples of the hydrophobic effect:
Droplet formation (like oil in spaghetti water).
Liposomes & Nanovesicles:
Man-made to deliver drugs more safely and effectively
Have hydrophobic membranes and hydrophilic cores.
If the drug is hydrophilic (water-loving) → it sits in the watery core.
If the drug is hydrophobic (water-fearing) → it hides inside the membrane part.
Used to encapsulate drugs for delivery.
Tell me difference between polar and non-polar groups
Non-polar bonds (hydrophobic): charges are evenly spread out, so no +/– ends.
Carbon (C) and Hydrogen (H) have similar electronegativity → no permanent dipole.
Polarity only occurs temporarily when electrons shift unevenly → induced dipole.
Polar bonds (hydrophilic): have a clear “+ end” and “– end.”
Oxygen (O) and Nitrogen (N) are highly electronegative.
Example: Alcohol (-OH)
Oxygen pulls electron density toward itself.
O becomes δ-, H becomes δ+.
This creates permanent dipole
How are functional groups related to amino acids?
Functional groups can be found as the R group of an amino acid
Go over the organic flash cards!!
What are the Nonpolar functional groups??
alkly groups
methyl (small)
ethyl (slightly larger and more nonpolar than methyl)
aromatic group
phenyl (found in phenylalanine) (very hydrophobic)
What are the Carbonyl-Containing functional Groups (C=O)?
aldehyde
Carbonyl bonded to H and an R group.
Reactive; can be oxidized to carboxylic acid.
Important in reducing sugar for another chemical!!
ketone
More stable, less reactive than aldehydes.
carboxylic acid
Can lose a proton → becomes negatively charged.
ester
Found in fruits (e.g., banana smell).
Remember: Esters = smells
acetyle
Found in acetyl-CoA, donor of acetyl groups in metabolism
anhydride (2 carboxylic acid)
difference between oxidation and reduction
Oxidation = usually loses hydrogen OR gains oxygen.
Reduction = usually gains hydrogen OR loses oxygen.
difference between nucleophile and electrophile
Nucleophile = giver (has extra electrons).
Electrophile = taker (wants electrons).
What are the Oxygen Single-Bonded Functional Groups?
alcohol (hydroxyl)
polar; decent nucleophile.
ether
relatively nonreactive, more hydrophobic than alcohols.
enol (he did not really talk about this)
What are the nitrogen containing functional groups?
Amine/Amino (NH3+-protonated)
Basic; usually carry a + charge at physiological pH.
Amide/Amido
Found in peptide bonds (amino acid linkages) (protein backbone)
Formed from carbonyl + amine.
Imine/Schiff base
formed during nucleophilic substitution reactions (forms in some enzyme mechanisms)
N-sub imine
Guanidinium
Found in arginine side chains.
basic
Imidazole
Found in histidine.
Can accept/donate H⁺, good for enzyme activity.
What are the Sulfur-Containing functional groups?
1. Thiols (–SH)
Like alcohols but with sulfur.
More polarizable → good nucleophiles.
2. Disulfides (R–S–S–R)
Formed from two thiols.
Important for stabilizing protein structure.
3. Thioesters
Like esters, but with sulfur.
Found in acetyl-CoA.
What are the phosphorus-containing groups?
phosphoryl
phosphanydride
mixed anhydride
Phosphorus-Containing Groups
Phosphoryl groups are central in biology.
Found in DNA, RNA, ATP.
Phosphoanhydride bonds (ATP):
Often called "high-energy," but really intermediate energy
Must be intermediate → allows ATP to be both synthesized(to made) and used as energy currency.
Acetyl-CoA has many of WHAT?
functional groups
What is titration?
slowly add one liquid to another to see when they balance (neutralize)