Batteries
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Primary Battery
Non-rechargeable battery, cheap and disposable e.g. Zinc Graphite, Alkaline, Lithium Iron Disulphide
Secondary Battery
rechargeable battery, designed for multiple used but with a limited lifetime such as Lithium-ion, Nickel-Cadmium, and Lead-Acid.
Anode
Electrode where oxidation occurs
Cathode
Electrode where reduction occurs
Gibbs Free Energy (Equation)
DG=-n*F*DE
Overpotential
Change in potential due to the loss or gain of electrons leading to a change in thermal level
Thermal Level
Average kinetic energy of a material
Standard Potential, E0
Potential above which a reaction will occur
Tafel Plot Relationship
i=a*exp(eta/b)
Arrhenius Equation
rate = A*exp(-Eact/RT)
Activation Potential, Eact
Potential above which reactants are converted to products, Eact=E-E0=eta
Butler-Volmer Equation
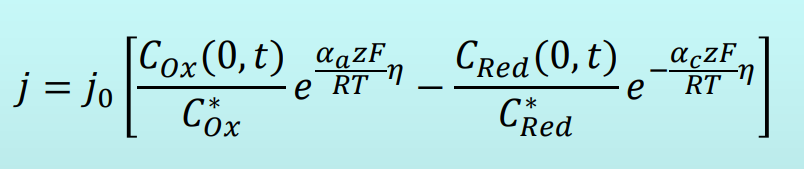
Butler-Volmer: Small Overvoltage
Linear, j=a+(a/b)*eta
Butler-Volmer: Intermediate Overvoltage
Tafel Plot: i=a*exp(eta/b)
Butler-Volmer: High Overvoltage
Mass transfer limit, Flick’s Law: j=flux=D* dCox/dx
Battery
Batch reactor with products and reactants contained
Extent of Reaction
opposite of state of charge
Sulphation
formation of insoluble lead sulphate on both electrodes blocking the active material
Acceptance Current
Proportion of current required to charge active material
Faradaic Efficiency
Ratio of acceptance to total current, charge or number of electrons
Self Balancing
When the state of charge of each cell balances out leading to the same charge throughout each cell
Memory Effect
Crystals form reducing the capacity of the battery, occurs when not fully discharged
C-rate
Current at which the battery fully discharges in one hour
Intercalation
When atoms diffuse between sheets of carbon during charge and discharge
Specific charge/discharge capacity
eta= x*F/n*M * 1000/3600 [mAh/g]
Cell Capacity
eta_cell = (eta+)*(eta-)/(eta+)+(eta-)
Cell Voltage
Ecell = Ec - Ea
Power Capacity
eta_w = eta_cell * E_cell
Tafel Equation
j=a*exp(eta/b)
Lead Acid Sulphation
During deep discharge solid and non-conductive PbSo4 forms on negative electrode leading to irreversible degradation
Lead Acid Trickle Charge
When charging batteries with imbalanced SoC in series, batteries with the highest SoC have a lower faradaic efficiency due to the side reaction of water electrolysis. This allows batteries with a lower SoC to catch up