(Not completed!) Chapter 14: Energy changes in chemical reactions
1/15
Earn XP
Description and Tags
Textbook flashcards on Chapter 14: Energy changes in chemical reactions
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
14.1 → Exothermic and endothermic reactions
Exothermic reactions:
In exothermic reactions, heat is released in order for the reactants to form products.
Energy of the reactants > (higher than) Energy of the products.
Practical example: Combustion. You can see that the reactants of ethanol are releasing heat to become the products (in the case of complete combustion, water and carbon dioxide):
C2H5OH (aq) + 3O2 (g) ---> 3H2O (l) + 2CO2 (g) ΔH = -1360 kJ
The heat of enthalpy (ΔH) is negative when the substance releases energy. You can imagine this as the reactant losing energy to form the product, thus, making the heat of energy negative.
Examples of exothermic reactions are:
Combustion of petrol and coal
Synthesis reactions (such as Fe + S, H2 +Cl2,)
Reactions of metals with water and acids (such as Li + H2O, Zn + H2SO4)
REactions of acids with bases and carbonates (such as H2SO + NaOH and CaCO3 + HCl)
Endothermic reactions:
Heat is absorbed in order for the products to form.
Energy of the reactants < (less than) Energy of the products
Practical example: melting ice. You can think of the ice as absorbing energy to become liquid water. This will have a chemical formula:
H2O (s) ---> H2O (l) ΔH = +6 kJ
The heat of enthalpy (ΔH) is positive when the substance is absorbing energy. You can imagine this as the reactant gaining energy, thus making the heat of enthalpy positive.
Some more examples of endothermic reactions include:
Decomposition of Cu(NO3)2 or CaCO3
Some precipitation reactions such as MgCl2 + Na2CO3
And photosynthesis
14.2 → Temperature, quantity of heat and heat capacity
Temperature vs. Heat → what’s the difference?
Temperature - is the average kinetic energy of particles, independent of the number of particles. It is the physical quantity we use to measure the degree of hotness and coldness of an object or substance.
Heat - is the total energy of all particles, energy of every particle is included, and is dependent on the number of particles.
If two objects are brought into contact, heat will flow from the hot object to the cold one until the temperature of the two objects is equal. Heat always flows from high → low temperature.
Quantity of heat
The amount of heat or quantity of heat is different from temperature: two objects can be the same temperature but contain two very different amounts of heat. It can then be concluded that:
The amount of heat energy is proportional to the mass of the substance involved
The amount of heat energy contained in equal masses of different substances depends on the nature of the substances involved
Say if you had a red, hot nail and a luke warm swimming pool, the swimming pool would in fact have more heat. This is because of the grand scale of particles present. While the nail may be increased in temperature, there are much less particles present, and therefore the swimming pool would have more heat.
Specific heat capacity
The specific heat capacity (symbol: c) of a substance is the amount of heat required to increase the temperature of unit of mass of the substance by exactly 1 degree Celsius/Kelvin
It is measured in Joules per kelvin per gram (J/gK) or Joules per kelvin per kilogram (J/Kkg)
The specific heat capacity of water is 4.18 J/gK
The measurement and calculation of heat changes is called calorimetry
Calculating quantities of heat
Formula: q=mcΔT
Where q: the quantity of heat
Where m: the mass of the substance
Where c: the specific heat capacity of the substance
WHere ΔT: is the difference in the initial and final temperatures of the substance
3 → Enthalpy
Enthalpy
Enthalpy is a measure of the total energy possessed by a substance or group of substances.
You can think of it as being mainly the chemical energy stored in a substance.
We are unable to measure the enthalpy of a substance, but we can measure the changes in enthalpy!
Changes in enthalpy
The change in enthalpy for a chemical reaction ΔH, is defined as the heat absorbed per mole of specified reactant or product when the reaction occurs at constant pressure.
Because most experiments we deal with occur at constant pressure (open to the atmosphere), the heat absorbed will be a direct measure of ΔH.
By ‘change in enthalpy’ it is meant the increase in enthalpy going from reactants to products
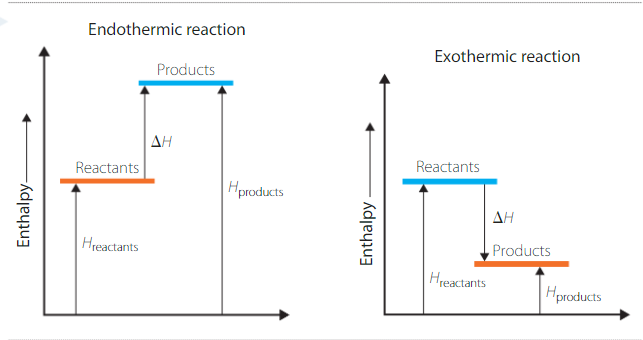
ΔH = enthalpy of products - enthalpy of reactants
For endothermic reactions, ΔH is positive (as it is “gaining” energy)
For exothermic reactions, ΔH is negative (as it is “losing” energy)
Changes in ΔH depend on the physical state (solid, liquid, gas or aqueous) of the reactants and products, and therefore in chemical energy contexts, we must always indicate the physical state of the substance involved in the chemical equation
Enthalpy changes in dissolution
The molar enthalpy of solution of heat or of solution, ΔH(soln), is the heat absorbed when one mole of the substance dissolves in a large excess of water
If heat is absorbed, ΔH(soln) is positive and the reaction is endothermic
If heat is released, ΔH(soln) is negative and the reaction is exothermic
For example:
C6H12O6(s) → C6H12O6(aq) ΔH(soln) = +11 kJ/mol
When an ionic substance dissolves, it breaks up into separate ions, which move freely and independently through the solution. The equation for dissolution of ionic substances shows this dissociation, for example potassium nitrate:
KNO3(s) → K+(aq) +NO3-(aq) ΔH(soln) = 35 kJ/mol
Dissolving ionic substances into aqueous solutions to form ions is called dissociation.
14.4 → Measuring enthalpy changes for reactions
Steps on how to measure the change in enthalpy
The change in enthalpy for a chemical reaction, ΔH, is defined as the heat absorbed per mole of specified reactant or product when the reaction occurs at constant pressure.
The general procedure for calculating molar enthalpy changes from experimental data is as follows:
Calculate the amount of heat released or absorbed, generally by using: q = mcΔT
Calculate the number of moles that reacted, by using: n = m/MM
Calculate heat released or absorbed per mole, by using: total heat released or absorbed/ number of moles that reacted
ΔH is positive when heat is absorbed, and negative when heat is released
14.5 → Heat of combustion
Molar heat combustion
The molar heat combustion of a substance is the heat liberated when 1 mole of the substance undergoes complete combustion with oxygen at a constant pressure of 100.0 kPa, with the final product being carbon dioxide gas and liquid water.