JC Science - Structure of the atom
0.0(0)
0.0(0)
Card Sorting
1/32
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
33 Terms
1
New cards
Atom
The smallest particle of an element that still has the properties of that element.
2
New cards
Molecule
When 2 or more atoms join together
3
New cards
Element
Substance made of 1 type of atom
4
New cards
Compound
2 or more different substance that are chemically combined
5
New cards
Mixtures
2 or more different substances that mingle but don't chemically combine
6
New cards
Why is it important to know the structure of the atom
-Tells us whether they are a metal or gas etc
-We can predict how they'll react to make a compound
-We can predict how they'll react to make a compound
7
New cards
Subatomic particles
-What atoms are made of
-Protons, neutrons, electrons
-Protons, neutrons, electrons
8
New cards
What are atoms made of?
-Protons
-Neutrons
-Electrons
-Empty space
-Neutrons
-Electrons
-Empty space
9
New cards
Diagram of an atom
10
New cards
a.m.u.
atomic mass unit
11
New cards
Protons (location, mass, charge)
-Location: nucleus
-Mass: 1 a.m.u
-Charge: +1
-Mass: 1 a.m.u
-Charge: +1
12
New cards
Neutrons (location, mass, charge)
-Location: nucleus
-Mass: 1 a.m.u
-Charge: 0
-Mass: 1 a.m.u
-Charge: 0
13
New cards
Electrons (location, mass, charge)
-Location: electron cloud
-Mass: 1/1836 a.m.u
-Charge: -1
-Mass: 1/1836 a.m.u
-Charge: -1
14
New cards
Why is the atom neutral overall
There is an equal number of positive protons + negative electrons so their charges balance
15
New cards
Atomic number
-The number of protons
-The number of electrons
-Always the smaller number
-The number of electrons
-Always the smaller number
16
New cards
Mass number
-Number of protons + neutrons combined
-Always bigger
-Always bigger
17
New cards
How do you find the number of neutrons?
mass number - atomic number
18
New cards
Number of Protons
the same as the Atomic number
.
.
19
New cards
muber of elecetrons
Same as atomic number
20
New cards
number of Neutrons
Mass No. - Atomic no
21
New cards
Isotopes
-Atoms of the same element that have different numbers of neutrons in the nucleus
-Different mass number
-Different mass number
22
New cards
Ions
Atoms that have lost or gained electrons
23
New cards
Anions
Elements which have gained electrons + are negatively charged
24
New cards
Cations
Elements which have lost electrons + are positively charged
25
New cards
Niels Bohr
-He designed a model for the atom
-He said the electron cloud was divided into shells
-He said the electron cloud was divided into shells
26
New cards
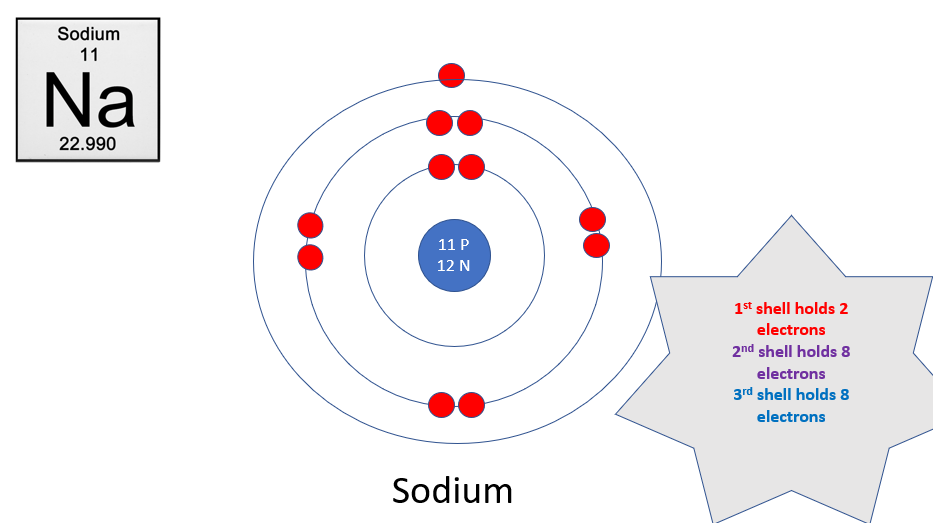
Electron shells (1 to 3)
1: max 2
2: max 8
3: max 8
2: max 8
3: max 8
27
New cards
Electron cloud
The space around the nucleus where electrons are held
28
New cards
What are electron shells
Energy levels of electrons
29
New cards
How does an element get a full outer shell
-Less than 4 take them away
-More than 4 then add up to 8
-4: add or take away
-More than 4 then add up to 8
-4: add or take away
30
New cards
What happens if the number of protons in an atom changes?
It becomes a different element
31
New cards
What happens if the number of neutrons in an atom changes?
It does not become a different element. It is an isotope
32
New cards
What happens if the number of electrons in an atom changes?
It does not become a different element. It is an ion
33
New cards
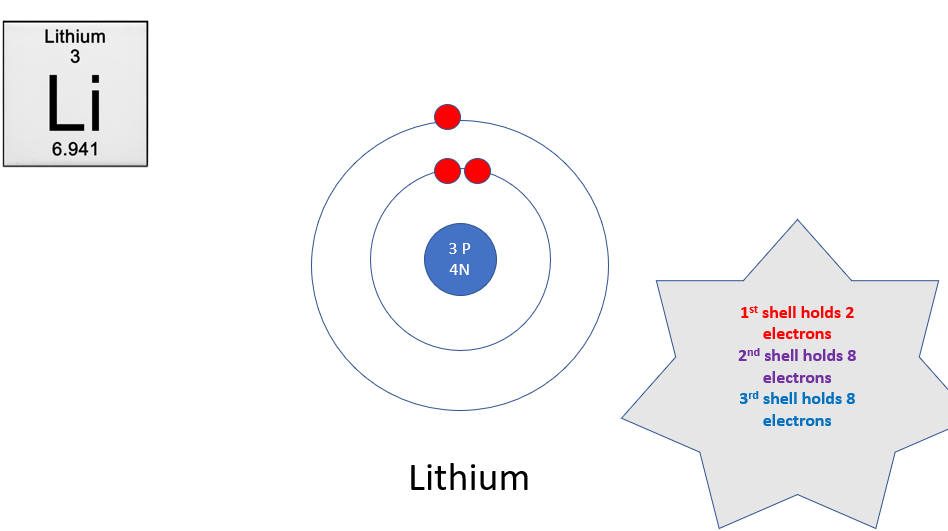
finish this diagram