Electronic Structure of Atoms
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms

Continuous Line Spectrum
When light passes through a prism it splits to give a continuous spectrum
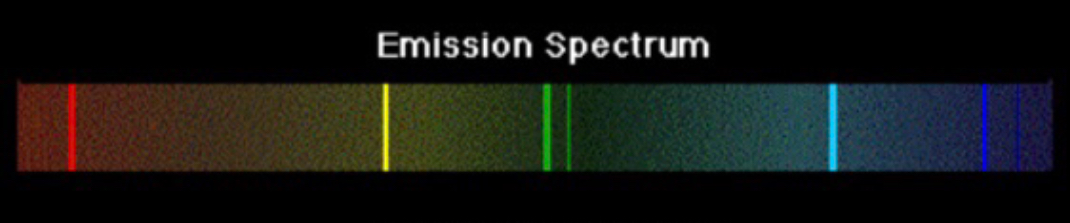
Emission Line Spectrum
Light is passed through a hot sample of gas. The emission line spectrum represents the light emitted by an atom when its electrons fall back to lower energy levels.
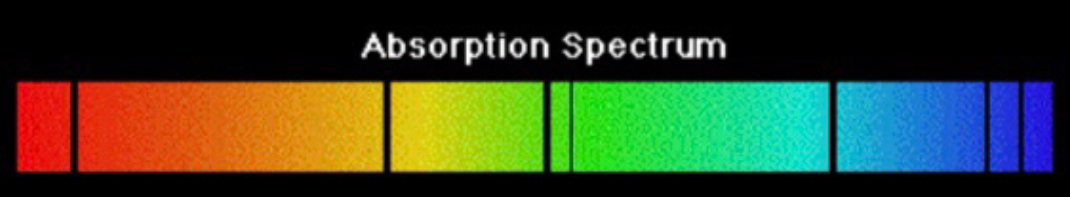
Absorption Line Spectrum
Light is passed through a cold sample of gas. The missing colours represent energy that entered the atom taken in by electrons.
Energy Level
The fixed energy value that an electron in an atom may have
Ground State of an Atom
One in which the electrons occupy the lowest available energy level
Excited State of an Atom
One in which the electrons occupy higher energy levels than those available in the ground state
Neils Bohr
Suggested the lines on the emission line spectrum represent energy levels with in an atom where electron could be found revolving around the nucleus in orbits
Photon of light explanation
In its ground state, electrons occupy the lowest available energy level. The electron can move (jump/become excited) to a higher energy level if it receives a certain amount of energy. The excited state is unstable and the electron falls back to a lower level. It emits the excess energy in the form of a photon of light.
Limitations to Bohr’s theory
Bohr’s theory only worked to explain the emission spectrum of Hydrogen.Bohr’s theory did not take into account the fact that an electron had a wave motion. Did not take into account the presence of sublevels.
Sublevel
A a subdivision of a main energy level and consists of one or more orbitals of the same energy
Atomic Orbital
A region in space where there is a high probability of finding an electron
Lyman series
When electrons fall from any energy level to the the first energy level (n=1) - ultraviolet light
Balmer series
When electrons fall from any energy level to the the second energy level (n=2) - visible light
Paschen series
When electrons fall from any energy level to the the third energy level (n=3) - infrared light
De Broglie
Stated that all moving particles move in wave motion
Heisenberg’s Uncertainty Principle
Its not possible to determine the exact position and speed of an electron at the same time only the possibility of locating an electron in a given place at any one time
Schrödinger
Developed a complex equation to calculate the location of an electron at any place at any one time. This equation revealed energy sublevels contain orbitals
Aufbau Principle
Electrons will occupy the lowest available energy sublevel
Hund’s Rule of Maximum Multiplicity
When two or more orbitals of equal energy are available, the electrons occupy them singly before filling them in pairs.
Pauli Exclusion Principle
No more than 2 electrons may occupy an orbital and they must have opposite spin.
Flame Tests: Sodium (Na)
Yellow
Flame Tests: Potassium (K)
Lilac
Flame Tests: Lithium (Li)
Crimson
Flame Tests: Copper (Cu)
Blue-green
Flame Tests: Barium (Ba)
Green
Flame Tests: Strontium (Sr)
Red