Catalysts + Measuring rate of reaciton
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
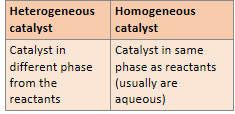
What state are heterogeneous and homogeneous catalysts in compared to their reactants respectively?
What is the mechanism of heterogeneous catalysts?
Reactants adsorb to surface of solid heterogeneous catalysts. -> Bonds in reactants weaken and break to form radicals which then react with each other to make products on catalytic surface-> products are released from the surface of the catalyst by desorption
How does increasing surface area of a heterogeneous catalyst affect rate of reaction?
Increasing surface area of this catalyst, increases rate of reaction -> more particles can react with catalyst at the same time
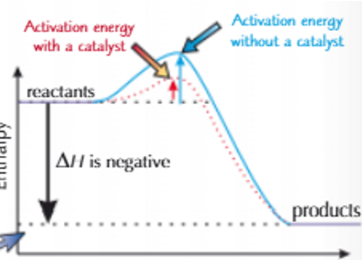
Draw the reaction profile diagram for a reaction using a heterogeneous catalyst and one without in an exothermic reaction
What if a heterogeneous catalyst has a very high adsorption strength?
It will not be suitable as desorption of products from catalytic surface would be too slow
What if a heterogeneous catalyst has a very low adsorption strength?
No significant chemical reactions can take place
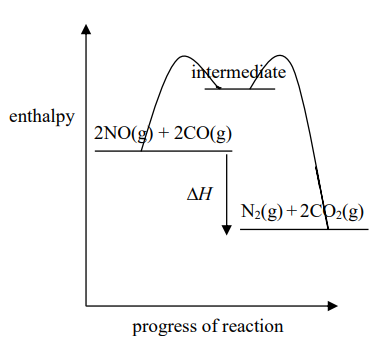
What is the mechanism of homogeneous catalysts?
Forms an intermediate species by reactants combining with the catalyst which react to form products. The catalyst is reformed again.
Draw the reaction profile diagram for a reaction using a homogeneous catalyst in an exothermic reaction
Draw the Maxwell Boltzman diagram for reactions with catalysts
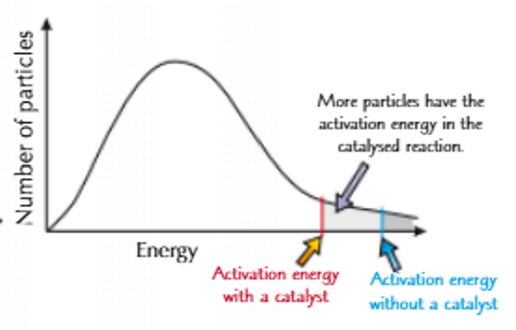
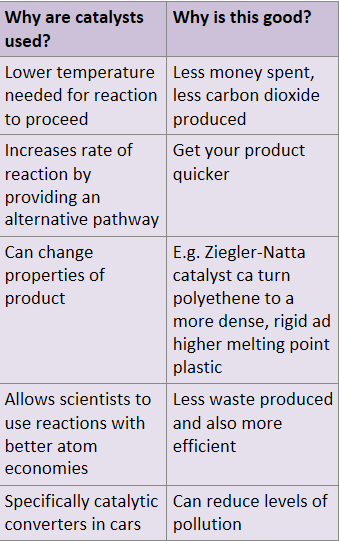
Why are catalysts used?
What are methods of measuring rate of reaction?


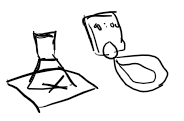
How do you measure rate via this method? Mention any problems you may come across and what precautions to take
Place cross on paper and time how long it takes for cross to disappear (precipitate to form)
Problem- difficult to knew when, can use same observer to reduce errors

How do you measure rate via this method?
Measure gas produced with a gas syringe. Measure this over a specified time
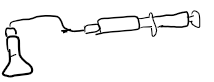
How do you measure rate via this method? Mention any problems you may come across and what precautions to take
Use on reactions that produce gas -> place reaction on balance and measure the mass of gas lost
Use a fume cupboard if gas is harmful or toxic
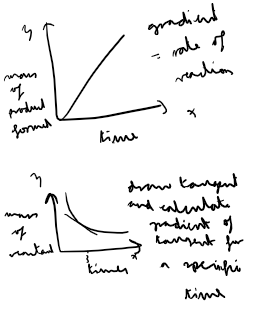
How do you calulate rate of reaction via a graph?