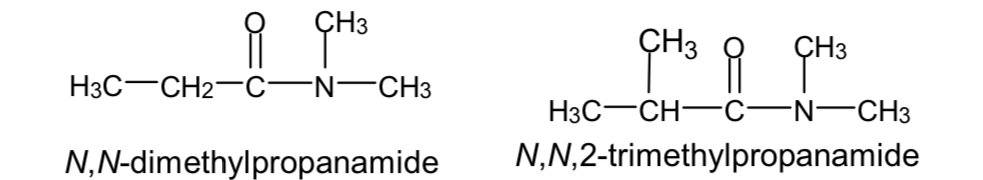Amides and Amines
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
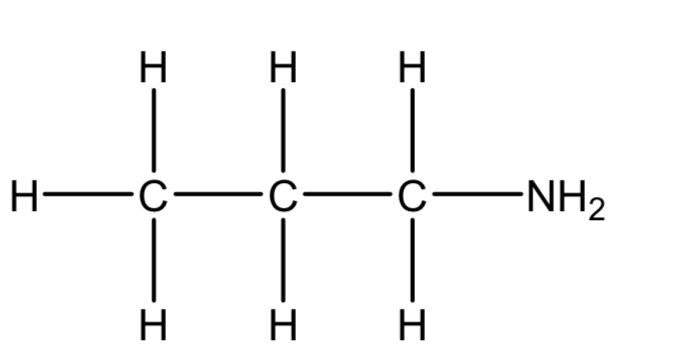
Name the compound
Propylamine or propan-1-amine
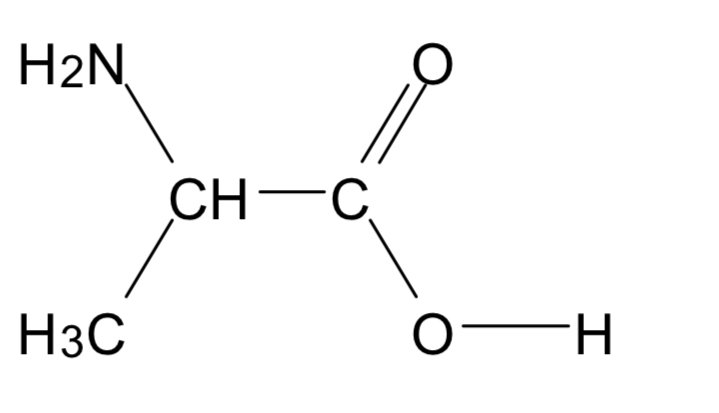
Name this compound
2- amino propane acid
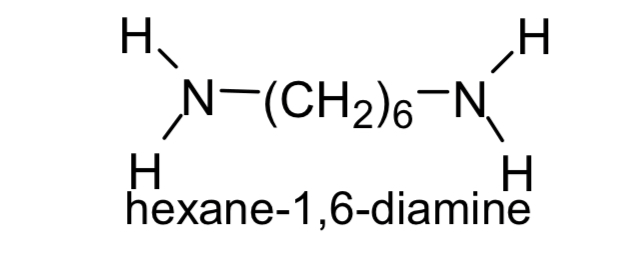
Name this
Hexan-1,6- diamine / 1,6 diaminohexane
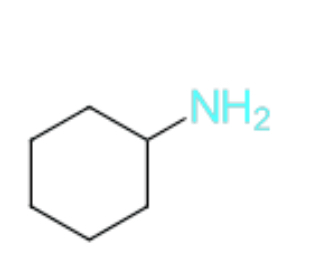
Name this compound
Cyclohexanamine
What are some properties of amines
fishy smell
Primary amines act as browsed Lowry base because of lp of e- on nitrogen which forms a dative covalent bond with H+ (accepts proton)
High bp bc amines form H bonds with water
What is the reaction of amines with to form alkaline solution and write the equation with ethyl amine and name the product
Lp on nitrogen accepts H+ to form water, amine acts as a base
CH3CH2NH2 + H2O → CH3CH2NH3 OH (ethyl ammonium hydroxide)
What is the reaction if amines with acid, what us formed, what happens in the reaction and write an equation using butylamine
forms a salt
Amine acts as a base, accepts H+ from acid to form ammonium salt
CH3CH2CH2CH2NH2 + HCl → CHCHCHCHNH3Cl
What is the reaction of amine with acyl chloride (ethanoyl chloride), what is formed, conditions, observations and equation wih ethyl amine?
room temp
Forms secondary amide
CH3COCl +2 CH3CH2NH2 → CH3CONHCH2CH3 (ethyllethanamide) + CH3NH3Cl (methyl ammonium chloride)
White solid
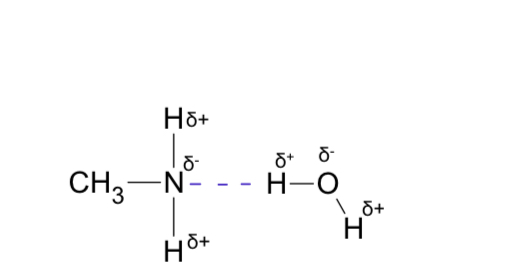
Draw methyl amine hydrogen bond with water
What is the reaction of amines with copper ions, what happens, what’s formed and observation and the equation of ethyl amine [Cu(H2O)6]2+
Blue precipitate
Amine acts as a base and accepts protons from water
2CH3CH2 +[Cu(H2O)6]2+ → [Cu(H2O)4(OH)2] + 2CH3CH2NH3+
What is the reaction of amines with halogenoalkanes and ammonia , what is formed and write the equation of methyl amine with chlormethane and ammonia (write both parts of the equation
forms secondary amine
CH3Cl + NH3 → CH3NH2 + HCl
Part 2: CH3NH2 + CH3Cl → (CH3)2 NH ( dimethylamine)+.HCl
Why are primary aliphatic amines stronger bases than ammonia and why are primary aromatic amines weaker bases than ammonia?
Primary aliphatic are stronger bc - alkyl pushes electrons towards nitrgen on amine so lone pair is more available to accept protons
Primary aromatic are weaker bc - lp of electrons on nitrogen delocalise with pi electrons on benzene so lone pair is less available to accept it
What 2 ways can primary aliphatic amines be prepared and give a disadvantage of both?
React with halogenoalkane and ammonia
Not effecirnt to prepare hgih yield of the amine bc further substitution reactions occur
Reduction of nitriles
Reduction of nitriles
2 step reaction so may have a low yield and potassium cyannide is toxic
How can primary aliphatic amines be prepared by reduction of nitriles, describe the 2 steps and
Step 1: convert halogenoalkane → nitrile with KCN, heat under reflux
Step 2: Reduce nitrile → amine with LiAlH4 using hydrogen, nickel catlyst
How can aromatic amines be prepared from nitroarenes? (conditions, reagant)
Reductioon reaction
Sn/ Fe and HCl
Heat
How can amides be formed?
React with acyl chlorides and ammonia which forms primary amine
React primary amine with acyl chloride to form amide
room temp
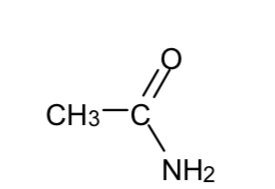
Name this compound
Ethanamide
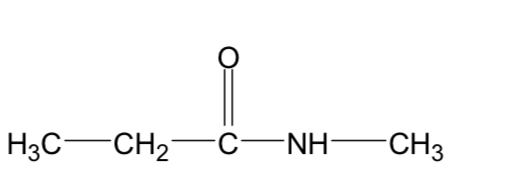
Name this compound
Methyl propanamide
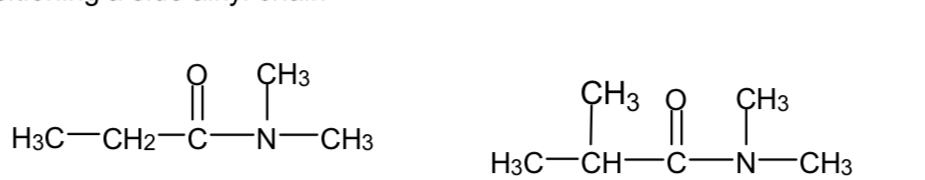
Name these two compounds