Action potentials: Hodgkin-Huxley intro, Hodgkin-Huxley model
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
What is an action potential?
An action potential is a rapid, temporary change in the membrane potential of a neuron, allowing for the transmission of electrical signals along axons.
What are the main phases of an action potential?
The main phases are depolarization, repolarization, and hyperpolarization (afterpotential).
How is an action potential initiated?
An action potential is initiated when a neuron receives a sufficient excitatory stimulus that depolarizes the membrane potential to reach the threshold level.
What is the role of voltage-gated ion channels in action potentials?
Voltage-gated ion channels open and close in response to changes in membrane potential, allowing Na⁺ and K⁺ ions to flow across the membrane, driving the phases of the action potential.
What occurs during the depolarization phase of an action potential?
During depolarization, voltage-gated Na⁺ channels open, allowing Na⁺ ions to rush into the cell, making the membrane potential more positive.
What occurs during the repolarization phase of an action potential?
During repolarization, voltage-gated Na⁺ channels inactivate, and voltage-gated K⁺ channels open, allowing K⁺ ions to exit the cell, returning the membrane potential to a negative value.
What occurs during the hyperpolarization phase?
Hyperpolarization occurs when K⁺ channels remain open longer than necessary, causing the membrane potential to become more negative than the resting potential.
What are refractory periods in action potentials?
Refractory periods are times following an action potential during which a neuron is less excitable. The absolute refractory period is when no new action potential can be initiated, and the relative refractory period is when a stronger stimulus is needed.
How does the Na⁺/K⁺ pump contribute to action potentials?
The Na⁺/K⁺ pump restores ion gradients after an action potential by actively transporting Na⁺ out and K⁺ into the cell, maintaining the resting membrane potential.
What factors influence the speed of action potential propagation?
Factors include axon diameter (larger diameters conduct faster), myelination (myelinated axons conduct faster), and temperature (higher temperatures increase conduction speed).
What is the Hodgkin-Huxley model?
The Hodgkin-Huxley model is a mathematical description of the ionic mechanisms underlying action potentials in neurons, developed by Alan Hodgkin and Andrew Huxley.
Why is the Hodgkin-Huxley model significant?
The Hodgkin-Huxley model was the first quantitative description of the action potential, providing insights into the roles of ion channels and setting the foundation for modern neuroscience.
What are the main components of the Hodgkin-Huxley model?
The model includes equations describing the conductances of sodium (Na⁺) and potassium (K⁺) channels, as well as a leak current, each contributing to changes in membrane potential.
How is ion conductance represented in the Hodgkin-Huxley model?
Ion conductance is represented by variable conductances gNa, gK, and gL (leak), each dependent on time and voltage, affecting the flow of ions across the membrane.
What role do voltage and time-dependent gates play in the Hodgkin-Huxley model?
Voltage and time-dependent gates control the opening and closing of Na⁺ and K⁺ channels, described by gating variables m,h, and n, which respond to changes in membrane potential.
What are the gating variables in the Hodgkin-Huxley model, and what do they represent?
The gating variables are mmm (activation of Na⁺ channels), hhh (inactivation of Na⁺ channels), and nnn (activation of K⁺ channels), representing probabilities of channel states.
What are the key equations in the Hodgkin-Huxley model?
The Hodgkin-Huxley equations describe changes in membrane potential (VVV) and ion channel conductances:
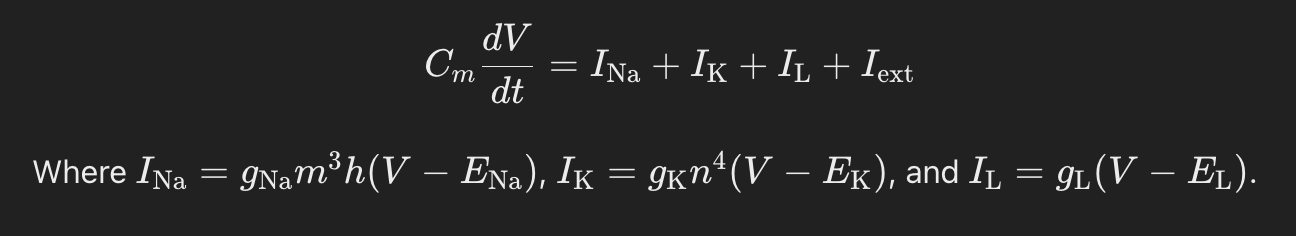
What role does membrane capacitance (Cm) play in the Hodgkin-Huxley model?
Membrane capacitance represents the cell's ability to store and separate charge, affecting the timing and dynamics of voltage changes during action potentials.
How are parameters estimated in the Hodgkin-Huxley model?
Parameters such as maximum conductances and equilibrium potentials are estimated from experimental data obtained by measuring ion currents in squid giant axons.
What are the applications of the Hodgkin-Huxley model?
The model is used to study neuronal excitability, simulate action potentials in computational neuroscience, and understand mechanisms of neurological diseases and drug effects.
What is the action potential threshold, and why is it important?
The action potential threshold is the critical level of membrane depolarization required to trigger an action potential. It ensures that only significant stimuli lead to neuronal firing, preventing random noise from causing signals.
What is the all-or-none principle of action potentials?
The all-or-none principle states that once the action potential threshold is reached, a full action potential is generated, and its amplitude is independent of the stimulus strength, as long as it surpasses the threshold.
What is saltatory conduction, and how does it enhance action potential propagation?
Saltatory conduction occurs in myelinated axons, where action potentials jump between nodes of Ranvier, increasing conduction speed and energy efficiency by reducing the area of depolarization.
How does the myelin sheath affect action potentials?
The myelin sheath insulates axons, preventing ion leakage and increasing conduction velocity. It allows for rapid signal transmission over long distances with minimal energy expenditure.
What happens to action potentials in demyelinating diseases?
Demyelination, as seen in diseases like multiple sclerosis, slows down or blocks action potential propagation, leading to impaired nerve signal transmission and neurological symptoms.
How was the Hodgkin-Huxley model developed?
The model was developed by Hodgkin and Huxley in the 1950s through experiments on the squid giant axon, which allowed them to measure ionic currents and characterize membrane conductance changes.
What is the voltage-clamp technique, and why was it crucial for the Hodgkin-Huxley model?
The voltage-clamp technique fixes the membrane potential, allowing researchers to measure ionic currents and isolate the contributions of specific ion channels, essential for developing the Hodgkin-Huxley equations.
How do gating kinetics influence the Hodgkin-Huxley model?
Gating kinetics describe the rate at which ion channels open and close in response to voltage changes. The model uses differential equations to represent these kinetics, determining channel conductance over time.
How are the gating variables represented mathematically in the Hodgkin-Huxley model?
The gating variables m,h,and n follow first-order differential equations where a and beta are rate constants dependent on membrane potential.
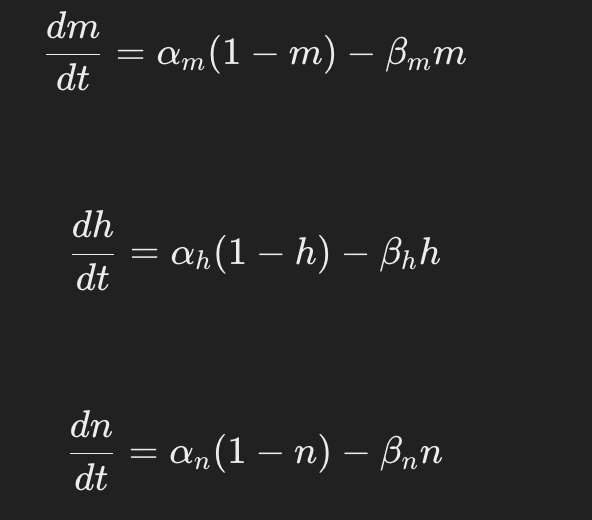
how do the parameters alpha and beta in the Hodgkin-Huxley model affect ion channel behavior?
The parameters alphaα and beta represent the voltage-dependent rate constants for opening and closing ion channel gates, influencing how quickly channels respond to changes in membrane potential.
What constitutes the total membrane current in the Hodgkin-Huxley model?
The total membrane current is the sum of ionic currents INa,IK ILI, plus any external current Iext applied to the membrane.
How do ionic conductances change during an action potential in the Hodgkin-Huxley model?
During an action potential, gNa rapidly increases as Na⁺ channels open, causing depolarization. gK then rises as K⁺ channels open, leading to repolarization. Conductance returns to baseline after the action potential.
What are time constants in the Hodgkin-Huxley model, and what do they signify?
Time constants (τ) describe how quickly gating variables approach their steady-state values. They influence the speed of channel opening and closing during an action potential.
What are some limitations of the Hodgkin-Huxley model?
The model does not account for complex neuronal structures, spatial variations in ion channel distribution, or interactions with other cellular components, limiting its application to simple axonal geometries.
How has the Hodgkin-Huxley model been extended in modern neuroscience?
Extensions include the incorporation of additional ion channels, synaptic dynamics, dendritic processing, and spatial modeling to simulate more complex neuronal behaviors.
How is the Hodgkin-Huxley model used in clinical research?
The model helps understand the mechanisms of neurological disorders, test hypotheses about ion channel mutations, and develop drugs targeting ion channels to treat diseases like epilepsy and cardiac arrhythmias.
How is the Hodgkin-Huxley model applied in computational neuroscience?
The model serves as a foundation for simulating neuronal activity in neural networks, aiding in the study of brain function, learning, and information processing.
What is the biophysical interpretation of the Hodgkin-Huxley equations?
The equations describe how voltage-gated ion channel dynamics and ionic currents produce changes in membrane potential, providing insight into the electrical behavior of neurons.
What influence has the Hodgkin-Huxley model had on modern neuroscience?
The model has profoundly impacted our understanding of neuronal excitability and action potentials, paving the way for quantitative approaches in neuroscience research and education.
What are future directions for research based on the Hodgkin-Huxley model?
Future research aims to integrate more biological details, such as genetic and molecular influences on ion channel behavior, and to apply the model to study complex neural circuits and brain disorders.