Reversable reaction and Kc constant
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What is Dynamic equilibrium
Rate of forward reaction= rate of backward reaction
How does Dynamic equilibrium look graphically
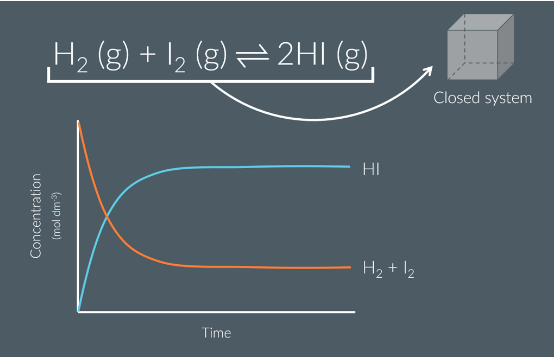
What system must dynamic equilibrium be in
Closed system
When is the equilibrium position to the right
When the concentration of reactants < concentration of Products
When is equilibrium position to the left
When the concentration of reactants > Concentration of Products
What are the factors that affect the equilibrium
Concentration
Pressure
Temperature
How does concentration affect the equilibrium
Increasing Concentration of reactants - Increases yeild of products - shifts to the right
How does tempature affect the equilibrium
Increasing temperature, moves equilibrium in the endothermic reaction
How does pressure affect the equilibrium
equilibrium position moves to the side with fewer gas moles
What is Le Chatiler’s Principle
When a factor that affects equilibrium is changed equilibrium moves to oppose that change
How does catalyst affect equilibirum
Increase the rate of forward and Backward equally
Do not affect equilibrium position
Why do Industries compromise conditions
Industries Want to produce maximum yield at the fastest Rate
Find the Kc equation for A + 2B ⇌ C + 3D
⟦C⟧⟦D⟧3 / ⟦A⟧⟦B⟧2
what table to use when only certain concentration known
ICE Table Reactant 1 Reactant 2 Product
Initial 0
Change Mole ratio
Equilibrium
What affect does pressure and concentration have on Kc
No affect
What affect doe Temperature have on Kc
Increase Temperature if forward reaction i endothermic , Value of Kc increases