QCHEM Chapter 11: Modern Atomic Theory
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
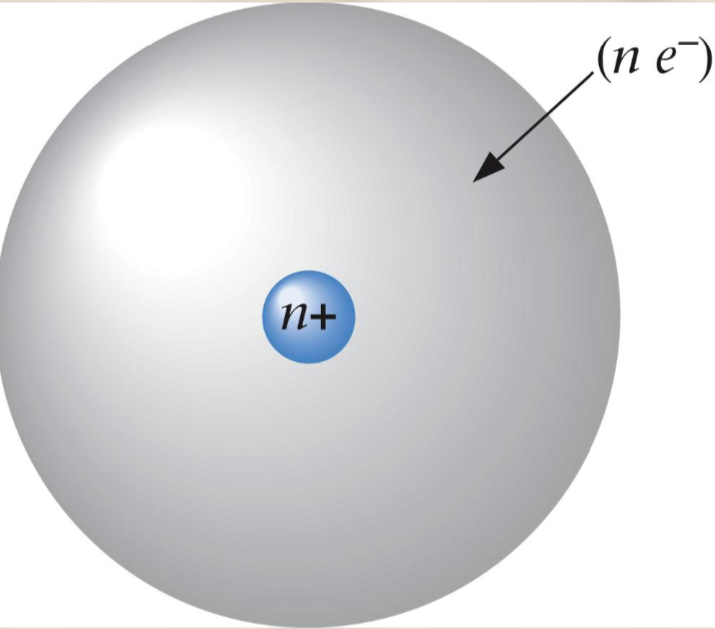
Rutherford’s atom
His nuclear model of the Rutherford atom has a small dense nucleus which is positively charged. The nucleus contains protons with a positive charge and neutrons with no charge. The remainder of the atom is mostly empty space with some electrons that have a negative charge. The nuclear charge is balanced by the presence of electrons moving in some way around the nucleus. The electrons race around a central nucleus at such high speeds that they act as WAVES OF ENERGY.
Electromagnetic radiation
Light can come in the form of a wave and as a stream of photons or packets of energy
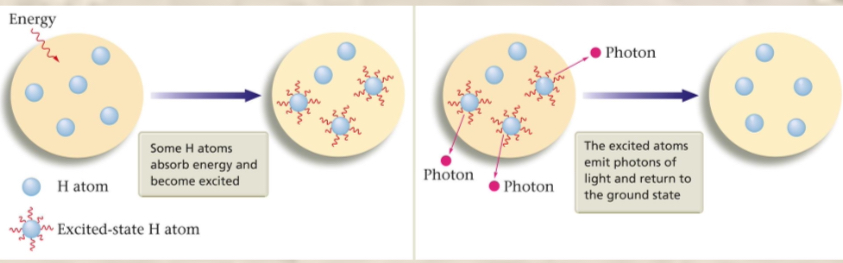
The energy levels of hydrogen
There are multiple states of an atom—“atomic states.” The excited state is when an atom has excess energy. The ground state is when an atom is in the lowest possible state or most stable configuration—there is no extra energy added to it. When a hydrogen atom absorbs energy from an outside source it enters and excited state as it has extra energy.
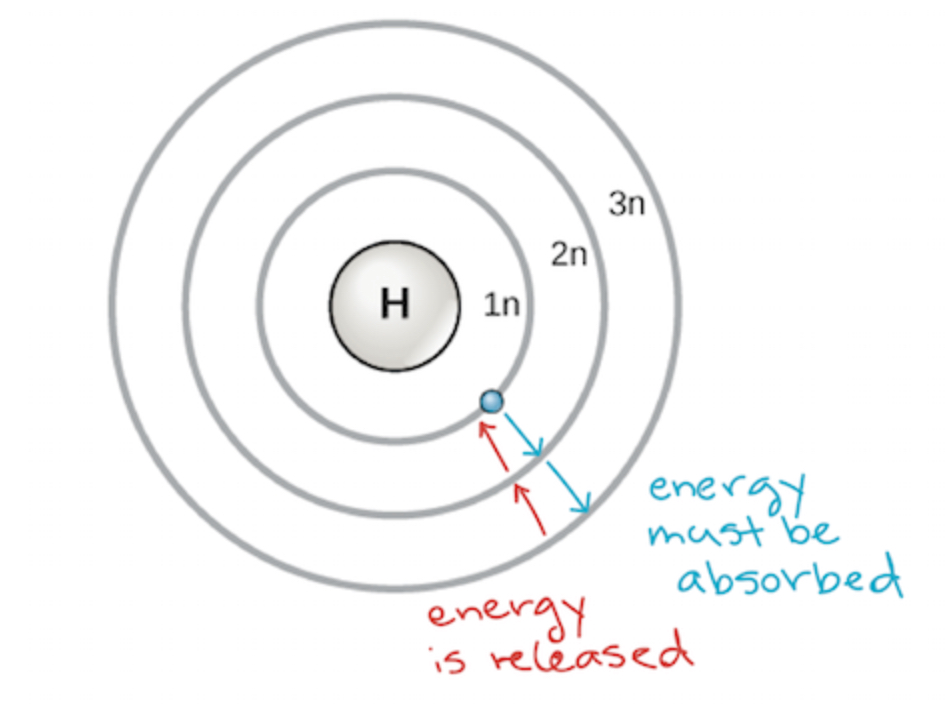
The Bohr model of the atom
According to Bohr, there are quantified energy levels in which electrons are said to have specific energy level where they are stored instead of being able to move within a range of energy levels. This goes along with the idea of orbits in which Bohr believed electrons move in this circular orbit and that electrons can jump between these energy levels by absorbing or emitting a photon of a particular wavelength. However, he was WRONG—electrons do not move in a circular orbit. His model only works for the hydrogen atom and does not apply to all atoms with many electrons and does not take into account dual wave-particle nature (he treated electrons purely as tiny particles not as waves that could make electrons exist in probability clouds).
The Wave Mechanical Model of the Atom—Schrödinger s model
In Schrödinger’s model, it was nothing like Bohrs, since Schrödinger believed that orbitals existed. Orbitals—probability of finding the electron within a certain space. However, this model gives no information about WHEN the electron occupies a certain point in space or HOW it moves.
The Hydrogen Orbitals
Orbitals don’t have sharp boundaries and chemists arbitrarily define an orbital’s size as the sphere that contains around 90% of the total electron probability (90% of where it is possible that the electrons of an atom can be). Hydrogen has discrete energy levels (similar to the floors of a building)—these are called principle energy levels and are labeled with whole numbers. Scientists propose tat atoms can have up to SEVEN principle energy levels of electrons around the nucleus. For instance, since hydrogen has one electron, that electrons are said can be in any seven principle levels.
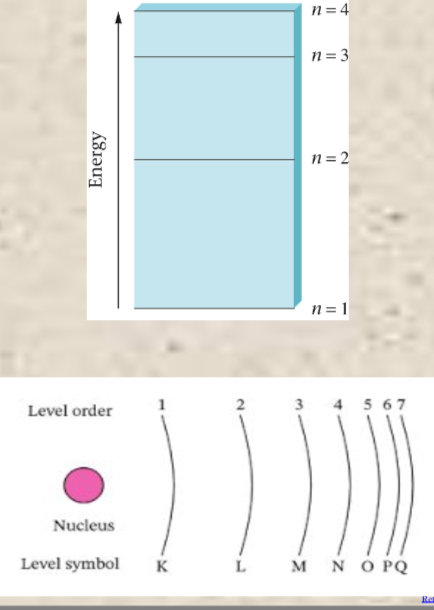
Principle Energy Levels
Each principle energy levels of electrons is divided into sub-levels labeled with numbers and letters and indicate the shape of the orbital. The higher the energy levels, the more orbitals it has or electrons it can hold. There are 2 electrons per orbital. As electrons push each other around, the electron orbital cloud forms different shapes (formed on the axes). Orbital labels—the number tells the principle energy level and the letter tells us the shape. S—spherical orbital. P—two-lobed orbital. X, y, or z subscripts on a p orbital tells u along which of the coordinate axes the two lobes lie to form its designated shape.
The Wave Mechanical Model: Further Development
The Bohr model was discarded since it does not apply to all atoms. Atoms beyond hydrogen have an equal number of protons and electrons—multiple electrons reacting. As a result, there was one more property that helped scientists determine how electrons are arranged—> its SPIN. This is how the electron spins (like how the earth spins on its axes)
Pauli Exclusion Principle
An atomic orbital can hold a max of 2 electrons and those 2 electrons must have opposite spins (opposite axes on which the electron orbitals spin). In an orbital diagram (box diagram) this is why the arrows face opposite directions—to show its spins.
Hund’s Rule
Every orbital in a sub level is singly occupied before it is doubly occupied. It has to fit each orbital in the sub-level before repeating the orbitals again.
Aufbau Principle
It states that electrons are filled into atomic orbitals in the increasing order of energy levels (lowest to highest) and not a random order to fill the levels. This is because atoms (like other things governed by the laws of physics) tend to take on the lowest energy, most stable configuration they can—> so electrons fill up the lowest energy levels before moving from the inside out.
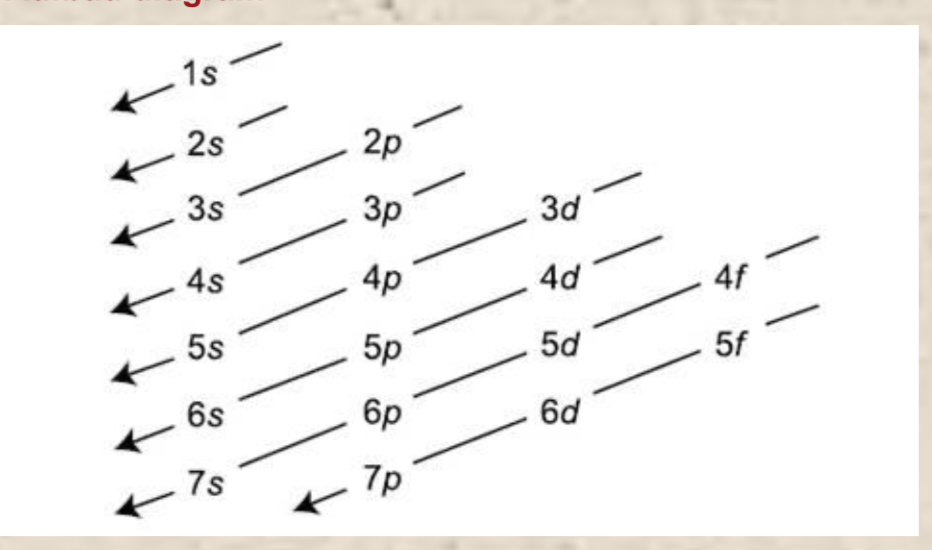
Electron Configuration
Full electron configuration—electron arrangement ex. 1s^1
Orbital diagram—orbitals are represented by boxes that are grouped by sub-levels and contain arrows to represent electrons and show the direction of their spins.
Abbreviated (shortened) Configuration—also called noble gas configuration. Write the previous noble gas in brackets (the symbol of the element) and then the last energy level valence electrons. (Note: it has to do with noble gases since they have 8 valence electrons and are therefore most stable)
Classifying Electrons
Core electrons are the electrons closet to the nucleus (inner electrons). However, we care about VALENCE ELECTRONS or the electrons in the outermost level or highest principle energy levels before moving of an atom. This is because valence electrons are the electrons that allow the atom to form bonds with other atoms. The elements in the same group on the periodic table have the same valence electron configuration, since elements with the same valence electron arrangement show very similar chemical behaviors—hence, they are grouped together.
Electron Configurations and the Periodic Table
Chromium and Copper are exceptions as it only fits ONE ELECTRON for each s level instead of 2 electrons like normal.
NOTE: For lanthanides and actinides, since they are supposed to be a part of the table, the f rows start with the element all the way on the left side. The last element in the separate row is what goes in the space on the periodic table (that element is a part of d). There are only 14 electrons for the f energy level!
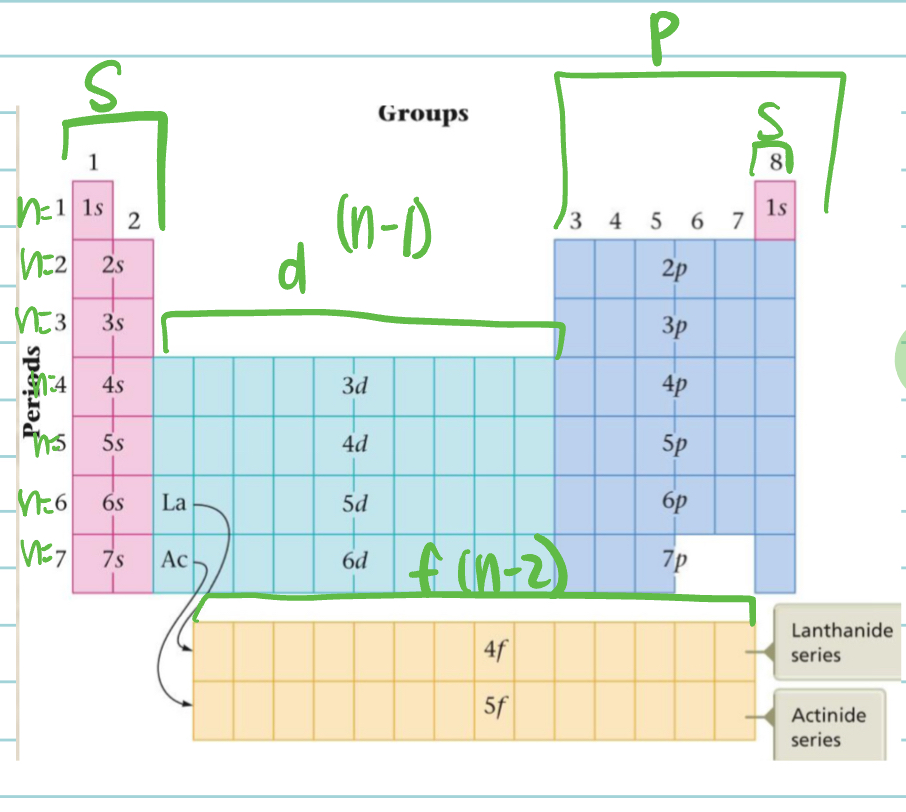
Orbital Filling Notes
Except for helium, the group numbers indicate the sum of the electrons in the s and p orbitals in the highest principle level that contains electrons or the VALENCE electrons
Metals and Nonmetals
Metals tend to lose electrons to form positive ions while nonmetals tend to gain electrons to form negative ions. The energy levels of nonmetals are bigger/expand, since they gain extra electrons.
Ionization Energy
The energy required to remove the highest-energy electron from a NEUTRAL atom. As you move across the period (left to right), the ionization energy increases because new electrons added, but not enough electron to create a new energy level so there is limited shielding (this means that there are no inner electrons that block the nucleus’s pull on the valence electrons.), so the nucleus’s pull on the valence electrons are stronger. This means it is harder to lose these electrons as they are bound very tightly. There are also more protons on the right of the periodic table, so the pull is stronger, so they need more energy to lose the electrons. For up to down on the period (going down on the groups) the ionization energy decreases, since there are additional electrons that create new energy levels. This means there are more inner electrons that create the shielding effect. Although there is an increase in protons, which strengthens the nucleus’s pull, however, there is shielding that creates a blockage for the valence electrons. As a result, it is easier for these valence electrons to be lost, so it doesn’t need as much energy to happen.
Atomic Radius
Atomic radius is the distance from the nucleus to the valence electrons. When going across the periodic table (left to right), atomic radius decreases especially because there is an increase in electrons. However, there is no energy shell added and there is limited shielding since there are no new energy levels so no increase in inner electrons to protect the pull of the nucleus. As a result, the nucleus’s positive charge, which will bring electrons closer to the center, decreasing the atomic radius. Also, there are more proton, which increases the charge so the atomic radius decreases and the effective nuclear charge increases. Fo up to down, the atomic radius increases. As the increase in electrons means that there is a gain in energy levels so there is more distance from the nucleus to the valence electrons. There is also an increase in protons but the shielding increases since there are more levels and therefore more electrons blocking the nuceus’s pll from the valence electrons (it doesnt pull the electrons in closer)
Ionic Size—Radius
Ionic size is the radius of an anion or cation (distance from the nucleus to outer edge of thee electron cloud. For anions, there are more electrons as it has a tendency to gain electrons, so the cloud expands (it may not adding a new level but the highest level expands. For cations, the ionic size decreases as it has a tendency to lose electrons. As a result, there is less electron-electron repulsion so the valence electrons can be pulled in more by the nucleus. Also, the number of protons remain the same so the nuclear charge is the same and therefore the electrons continue to be pulled into the center so the radius gets smaller.
Isoelectronic series
This is for ions that are derived from elements in different groups, á comparison of size is only possible in an isoelectronic series. It is a series of ions containing the same number of electrons. Note: the larger the atomic number, the smaller the ion because a higher atomic number means more protons, therefore the pull of the nucleus is stronger so the atom is also smaller.