Orgo I: Chapter 7: Alkenes: Structure and Reactivity
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
unsaturated
Fat with less than the maximum number of hydrogens
degree of unsaturation formula
(2+2C + N - X - H)/2
Step One in Naming Alkenes
Name the parent hydrocarbon (longest carbon chain with a double bond)
Step Two in Naming Alkenes
Number the carbon atoms in the chain (beginning at the end nearest to the double both or if both are equidistant, nearest the first branch point)
Step Three of Naming Alkenes
Write the full name (list substituents alphabetically, number the position of the double bond(s))
For cycloalkenes where does numbering start
around the double bond
ethylene
ethene
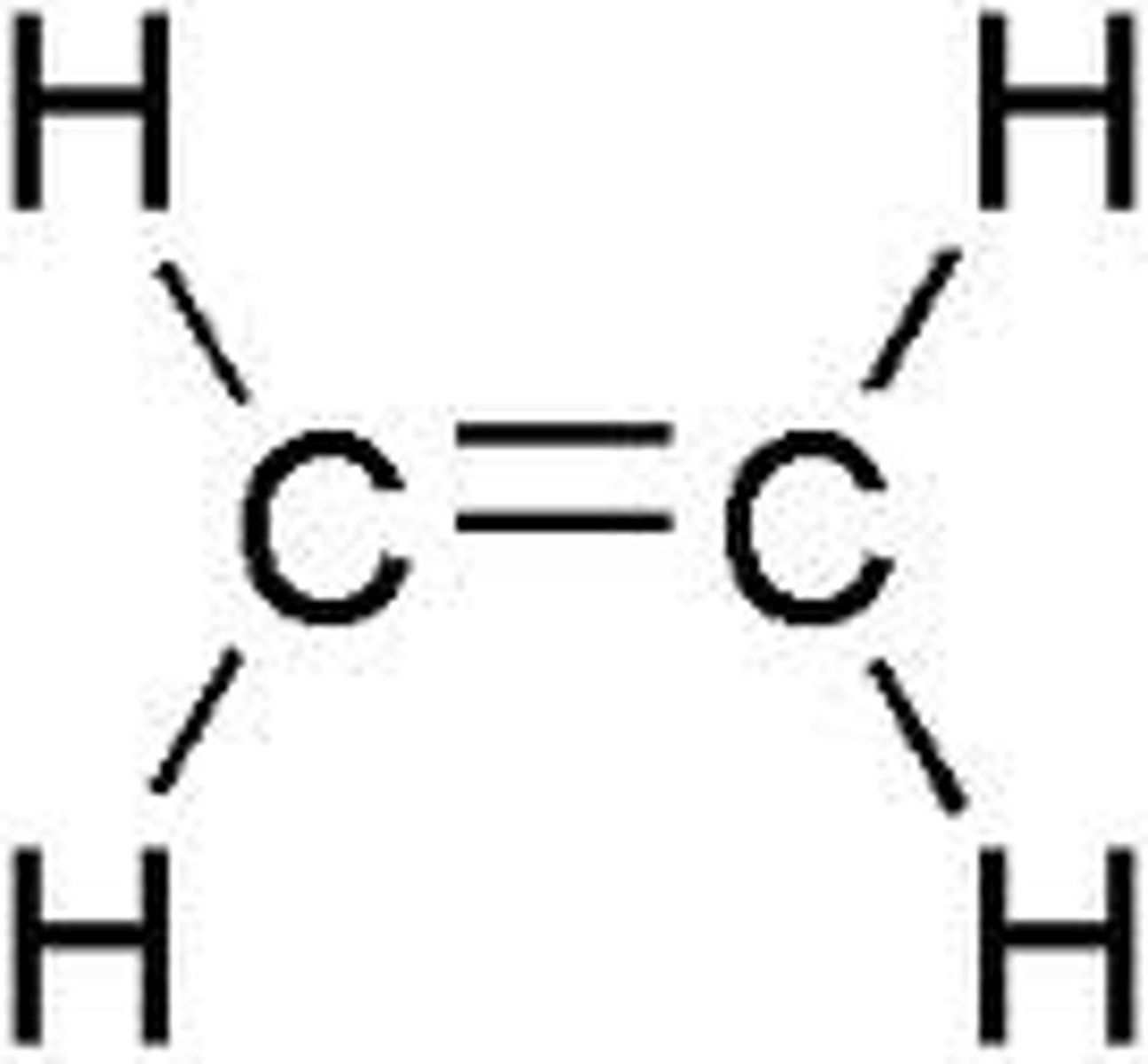
Propylene
propene
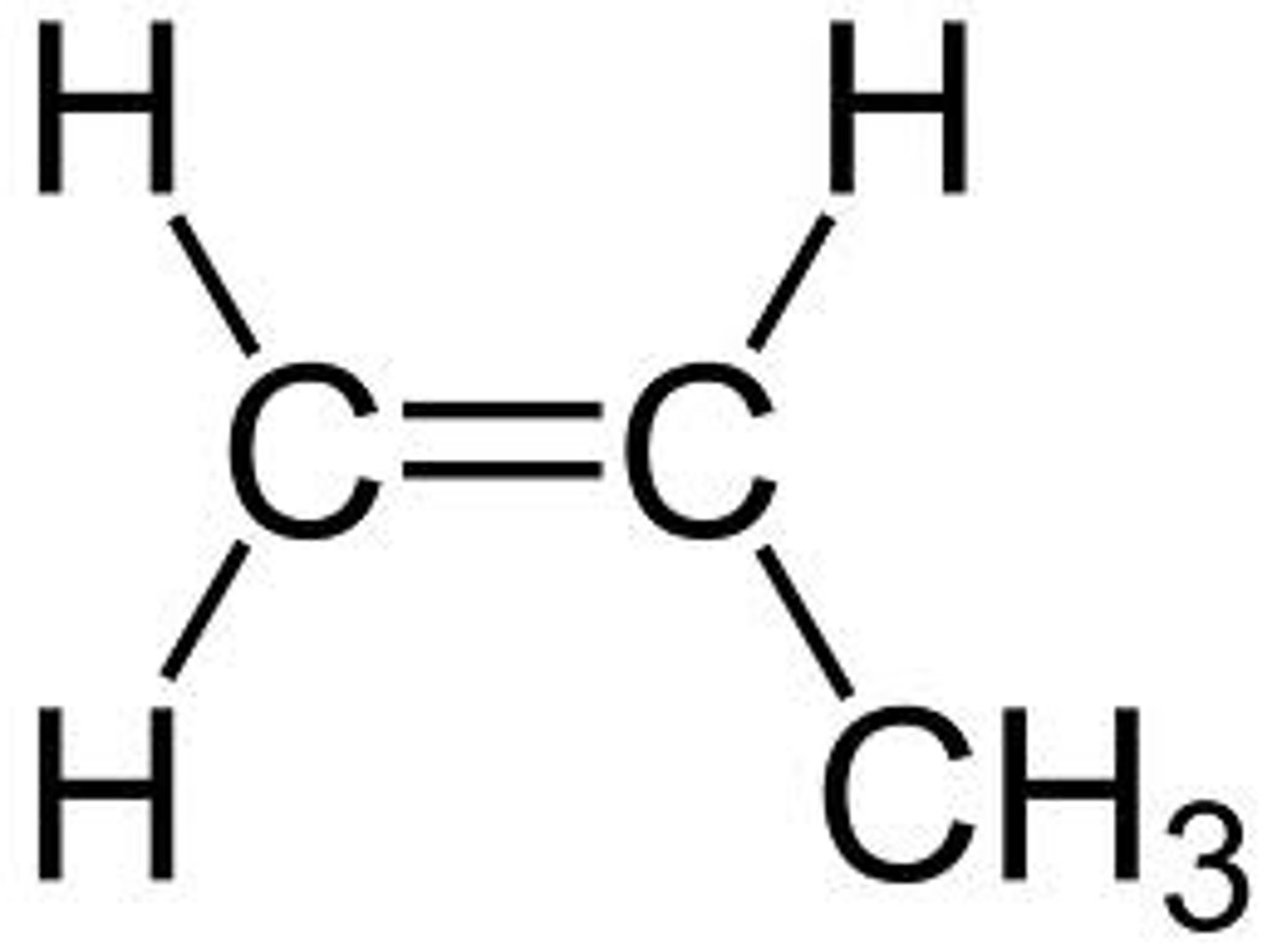
isobutylene
2-methylpropene
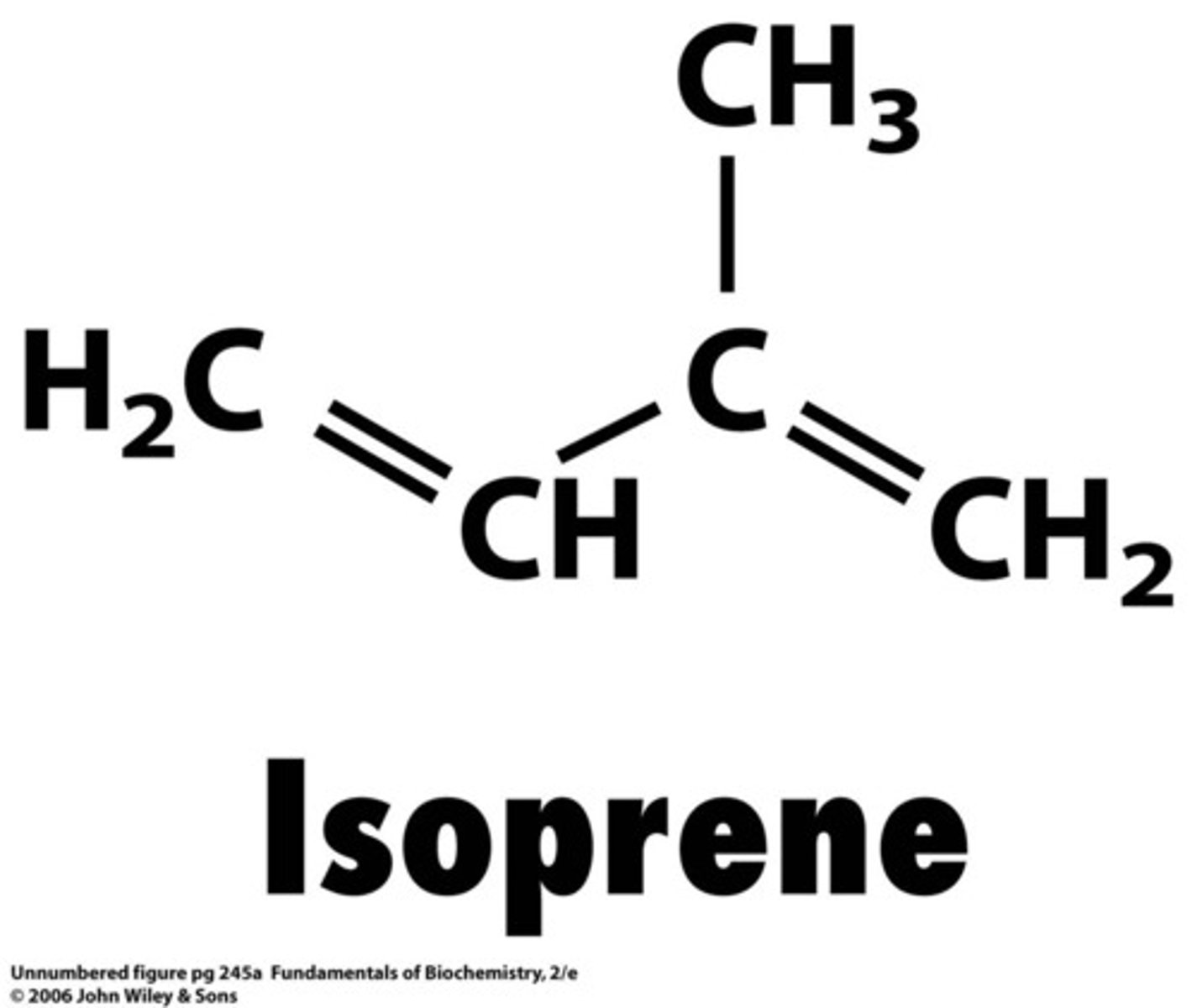
isoprene
2-methyl-1,3-butadiene
what is the phrase for remembering the Z geometry
ze zame zide
are cis alkenes more or less stable than trans and why?
less stable due to steric strain
are monosubstituted alkenes more or less stable?
less stable tetrasubstituted is most stable
hyperconjugation
Stabilizing interaction between a p orbital and properly oriented C-H sigma bonds on neighboring carbons that are roughly parallel to the p orbital
which is stronger sp3-sp3 bond or sp3-sp2 bond and what is the impact on stability?
sp2-sp3 is somewhat stronger so a higher ratio of sp3-sp2 bonds are more stable
do alkenes behave as nucleophiles or electrophiles in polar reactions
nucleophiles donating an electron pair from the double bond
electrophilic addition reaction
hydrogen atom on electrophile H-X attacked by electrons from nucleophilic double bond forming a new C-H bond leaving the other carbon C+ and leaving X-, the X- donates an electron pair to the C+ yielding a neutral addition product
which molecules can successfully complete electrophilic addition to alkenes and with what catalyst
HCl reactant, ether solvent, (HI) KI reactant, H3PO4 solvent, H20 reactant, H2SO4 solvent
regiospecific
only one of two possible directions of addition occurs
Markovnikov's Rule
H attaches to carbon with fewer alkyl substituents and X attaches to carbon with more alkyl substituents, also the more highly substituted carbocation is formed as the intermediate
Hammond Postulate
the transition state is more similar in structure to the species to which it is more similar in energy
hydride shift
the shift of a hydrogen atom and its electron pair between neighboring carbons