alkyne reactions
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
Preparation of alkynes: mechanism
Two eliminations (halogen and LG); aqueous workup to reprotonate alkynide ion
Preparation of alkynes: intermediate
alkynide ion
Preparation of alkynes: reagents
3 equivalents of strong base (NaNH2, NH3)
Preparation of terminal alkynes: reagents
1.) xs NaNH2 / NH3
2.) H2O
Preparation of alkynes: results
Alkyl halide/dihalide → alkyne
alkyl halide
R-X
Catalytic hydrogenation: stereochemistry
syn addition
Catalytic hydrogenation: mechanism
2 equivalents of H2 per pi bond
Catalytic hydrogenation: reagents
H2, Lindlar's catalyst/Ni2B
Catalytic hydrogenation with poisoned catalyst results
Alkyne → cis alkene
Catalytic hydrogenation (without poisoned catalyst) results
Alkyne → alkane
Catalytic hydrogenation: intermediate
alkene that cannot be isolated
Dissolving metal reduction: stereochemistry
anti addition (trans alkene)
Dissolving metal reduction: reagents
Na, NH3
Dissolving metal reduction: results
Internal alkyne → trans alkene
Hydrohalogenation: regiochemistry
Markovnikov
Hydrohalogenation: stereochemistry
racemic; proceeds via more stable, secondary vinylic carbocation OR termolecular
termolecular
hydrohalogenation; no vinylic carbocation; positive charge on more substituted position
Hydrohalogenation: mechanism
1st equ: trp bond attacks H, sigma bond attacks X (PT); halogen anion attacks pos charge on intermediate (NA)
2nd equ: alkene attacks HX; anion attacks pos charge
OR transition state
Hydrohalogenation: reagents
HX
xs HX
Hydrohalogenation: 1 equivalent results
Alkyne → halogen on more substituted C on alkene
Hydrohalogenation: excess reagent results
2 halogen on same C on alkane (geminal dihalide)
Hydrohalogenation: intermediate
vinylic carbocation
Hydrohalogenation: HBr + peroxides regiochemistry
anti-Markovnikov
Hydrohalogenation: HBr + peroxides stereochemistry
E and Z isomers
Hydrohalogenation: HBr + peroxides mechanism
radical
Hydrohalogenation: HBr + peroxides reagents
HBr, ROOR
Hydrohalogenation: HBr + peroxides results
Alkyne → Br terminal position
Acid-catalyzed hydration: regiochemistry
Markovnikov
Acid-catalyzed hydration: stereochemistry
No control
Acid-catalyzed hydration: intermediate
enol
Acid-catalyzed hydration: reagents
H2SO4, H2O, HgSO4
Acid-catalyzed hydration: results
Alkyne → ketone
Acid-catalyzed hydration used only for what?
terminal and symmetrical alkynes
Hydroboration-oxidation / base-catalyzed tautomerization: regiochemistry
anti-Markovnikov
Hydroboration-oxidation / base-catalyzed tautomerization: stereochemistry
syn addition
Hydroboration-oxidation / base-catalyzed tautomerization: intermediate
enolate ion (resonance-stabilized anion; cannot be isolated)
Hydroboration-oxidation / base-catalyzed tautomerization: results
Alkyne → aldehyde
Hydroboration-oxidation / base-catalyzed tautomerization: reagents
1.) BH3·THF or 9-BBN
2.) H2O2, NaOH
halogenation: regiochemistry
markovnikov
halogenation: stereochemistry
anti addition/trans isomer; E isomer with one equivalent; achiral
halogenation: xs reagents
xs X2
halogenation: one equivalent reagents
X2, CCl4
halogenation: two equivalents results
tetrahalide
halogenation: one equivalent results
2 halogen trans alkene (dihalide)
halogenation: XS reagent results
4 halogen on alkane
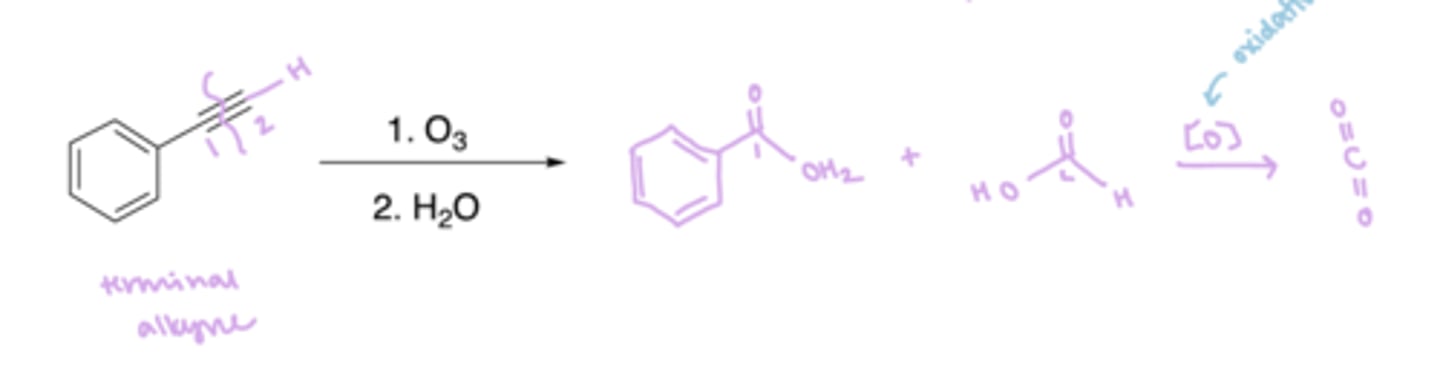
ozonolysis: reagents
1.) O3
2.) H2O
ozonolysis: internal alkyne results
2 carboxylic acids
ozonolysis: terminal alkyne results
1 carboxylic acid, 1 carbon dioxide
alkylation: mechanism
Forming C-C bonds (alkyl halide electrophile)
1.) terminal alkyne deprotonated by strong base
2.) deprotonated alkyne attacks carbon of methyl or alkyl halide (NA)
3.) sigma bond to halogen (LLG)
alkylation: reagents
1.) NaNH2
2.) XI
alkylation: intermediate
alkynide ion
alkylation: results
Elongated carbon chain; installation of alkyl group; two terminal protons = alkylation twice (separate)
alkylation restriction
methyl or primary alkyl halides only
dialkylation: reagents
1.) NaNH2
2.) Me/EtI
dialkylation: results
Addition of two alkyl groups to each side
mechanisms need to know with arrows
hydrohalogenation (3), alkylation, preparation of alkyne, HBO, ACH
Mechanisms with regiochemistry
ACH, HBO, hydrohalogenation, halogenation
Mechanisms with syn stereochemistry
HBO, catalytic hydrogenation
Mechanisms with anti stereochemistry
halogenation, dissolving metal reduction
stepwise mechanisms
HBO, ozonolysis, preparation of terminal alkyne, dialkylation alkyne, alkylation