AQA A level Chemistry - Required practicals
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
39 Terms
Describe a method to investigate how the rate of reaction changes with temperature
Disappearing cross: Change in the rate of reaction of sodium thiosulphate with hydrochloric acid as temperature is changed.
Na2S2O3 + 2HCl --> 2NaCl + SO2 + S + H2O
1. Add 10cm of 1mol of HCl to 'acid' tube. Place tube into container with no cross underneath
2. Use a measuring cylinder add 10cm of 0.05mol sodium thiosulphate solution to second tube with cross underneath. Place a thermometer in this tube.
3. Record start temp
4.Add 1cm of HCl to the sodium thiosulphate, start timing
5. Look down the tube from above and stop timer when the cross has dissapeared
6. Record the final temp of the sodium thiosulphate solution
7. Pour contents of tube into stopper bath
8. Boil a kettle and add hot water to the container (no hotter than 55).
9. Measure another 10cm of 0.05mol sodium thiosulphate solution into a clean 'acid' tube with cross underneath
10. Leave tube to warm up for 3min
11. Repeat steps 4-8 with different temps (room, 25, 35, 45, 55)
Safety considerations - RP (3) Change in temp on rate of reaction
-use plastic container to minimise release of toxic sulfur dioxide (fume cupboard could be used instead)
-eye protection, lab coat, gloved as HCl is an irritant
Why must a stop bath be used - RP (3) Change in temp on reaction
Acid and sulfur dioxide can be neutralised at any point during the experiment.
Make 250cm3 of a 2.00 moldm-3 solution of sodium hydroxide
(standard solution RP 1)
1) Calculate moles of sodium hydroxide using
moles = c x v
2.00 x 0.250 = 0.500 moles
2) Calculate grams of sodium hydroxide needed using
mass = mr x moles
40 x 0.500 = 20.0g
3) Place a weighing boat on a digital balance. Weigh out the required mass of solid (NaOH) and tip into beaker
4) Reweigh the weighing boat on a digital balance as it may still contain traces of NaOH
5) Subtract mass of boat from mass of boat + NaOH, to find precise mass of NaOh in beaker
6) Use wash bottle to make sure all of NaOH is in beaker, rinsing the sides ect.
7) Stir until solid is completely dissolved
8) Transfer solution from beaker into 250cm3 volumetric flask using funnel
9) Rinse funnel using wash bottle to make sure all NaOH is in flask
10) Top up flask to correct volume (bottom of meniscus on line) with more distilled water, add drop by drop when close to line
11) Stopper the flask and turn upside down 5x to mix
Standard solution complete
Titration RP 1
1) Rinse burette with acid you will use so that no water dilutes your solution and gives an inaccurate concentration
2) Fill burette with your standardised solution of acid
3) Use pipette filler to measure set volume of alkali sucked up into pipette (usually 25cm3)
4) Transfer alkali into conical flask
5) Add a couple drops in indicator eg methyl orange to conical flask
6) Take an initial reading of volume in burette
7) Do a rough titration, add acid until colour change of alkali
8) Record final volume on burette
8) Minus initial - final = rough titre
9) Use this to know when to begin dropwise when doing accurate titrations
10) Repeat accurate titrations until results are concordant (0.1 apart)
Required practical 4 Test 1
Describe a method, and state any observations, to test for the presence of group 2 metal ions using sodium hydroxide
1. Add barium chloride to a test tube
2. Add sodium hydroxide to tube and mix well, record any observations
3. Continue to add the sodium hydroxide until its in excess, record any observations
4. Repeat test with calcium bromide, magnesium chloride and strontium chloride
Results initially: all group 2 ions show no visible change (colourless solution)
Results with NaOH: BaCl2 remains colourless, all others show a slight white precipitate
Results with excess NaOH: BaCl2 remains colourless, CaBr SLIGHT white precipitate, MgCl white precipitate, SrCl2 SLIGHT white precipitate
Required practical 4 Test 2
Describe a method, and state any observations, to test for the presence of group 2 metal ions using sulphuric acid
1. Add barium chloride to a test tube
2. Add sulphuric acid to the tube and mix well, record any observations
3. Continue to add this sulphuric acid until its in excess, record any observations
4. Repeat test with calcium bromide, magnesium chloride and strontium chloride
Result with H2SO4: BaCl2 white precipitate, CaBr2 SLIGHT white precipitate, MgCl2 slight white precipitate, SrCl2 white precipitate
Results with excess H2SO4: BaCl2 white precipitate, CaBr sSLIGHT white precipitate, MgCl2 colourless solution, SrCl2 white precipitate
Required practical 4 Test 3
Describe a method, and state any observations, to test for the presence of ammonium ions
1. Add ammonium chloride to a clean test tube
2. Add NaOH to the test tube and mix
3. Warm the mixture in a water bath
4. Test the fumes released from solution by using forceps to hold a piece of damp red litmus paper in the test tube mouth
Result: ammonium ions turn the red litmus paper blue
Required practical 4 Test 4
Describe a method, and state any observations, to test for the presence of hydroxide ions in an aqueous solution (group 7 anions)
1. Test 1cm depth of solution in a test tube with red litmus paper, record observations
Results: NaOH with turn red damp litmus paper blue
Required practical 4 Test 5
Describe a method, and state any observations, to test for the presence of carbonate ions (group 7 anions)
1. Add an equal volume of dilute HCl to sodium carbonate solution in a test tube
Na2CO3 + 2HCl ---> 2NaCl + H2O + CO2
2. Use a delivery tube to transfer any gas produced into a second test tube containing calcium hydroxide (limewater)
Ca(OH)2 + CO2 ---> CaCO3 + H2O
Results: carbonate ions will turn limewater cloudy if present
Required practical 4 Test 6
Describe a test, and state any observations to test for the presence of sulphate, SO42-, ions (group 7 ions)
1. Add an equal volume of dilute HCl to the solution
2. Add an equal volume of barium chloride to the solution
3. Barium sulphate formed (white precipitate)
4. Add a small volume of dilute HCl
Results: as the precipitate does not dissolve, sulphate/hydrogensulphate ions are present
MgSO4 + BaCl2 ---> BaSO4 + MgCL2
Barium chloride is harmful wear gloves
Required practical 4 Test 7
Describe a method, and state any observations, to test for the presence of halide ions: KCL, KBR, KI
1. Add a small volume of dilute nitric acid to the solution of potassium chloride
2. Add silver nitrate to the solution, record any observations
3. Swirl the solutions and half them
4. To one half, add an excess of dilute aqueous ammonia solution, observe observation
5. To the other half (in a fume cupboard) add an excess of concentrated ammonia solution, record any observations
6. Repeat with KBR and KI
Results:
Silver nitrate - KCl white precipitate, KBR cream precipitate, KI yellow precipitate
Dilute ammonia - KCl colourless solution, KBr cream precipitate, KI yellow precipitate
Concentrated ammonia - KCl colourless solution, KBr, colourless solution, KI yellow precipitate
Required practical 4 Test 8
Describe a method, and state any observations, to test for group 7 ions using solid salts KCl, KBr, KI
1. Add small spatula of solid KCl to a clean test tube
2. Working in a flame cupboard, add a few drops of conc. H2SO4, record any observations
3. Test any gas evolved with blue litmus paper, record observations
4. Repeat with KBr and KI
Results:
Conc sulfuric acid - KCl white steamy fumes, KBr orange fumes, KI purple flames
Blue litmus paper - KCl, KBr, KI all turn red
Required practical 2a
Describe how to measure the enthalpy change when copper sulfate dissolves in water. Specify how you would handle the data in order to calculate a value of ΔH for this process
-Measure 50cm3 of water using a measuring cylinder
-Transfer to a polystyrene cup with lid
-Measure approximately 4g of copper sulfate using a balance
-Use a thermometer to take the temperature of water each minute for 3 minutes (establishes an initial temp)
-Add the copper sulfate at minute 4 (do not record a temp)
-Stir
-Continue to record the temperature each minute for up to 15 minutes
-Plot a temp (Y axis) against time (X axis), extrapolate to point of addition to determine Δt
How to reduce uncertainty in a mass measurement
-Use a larger mass
-Use a balance with a greater resolution
Required practical 2a
Why might an experimental value for an enthalpy change be different to a theoretical value?
-Heat loss to surroundings
-Incomplete combustion
Required practical 2a
How to prevent heat loss to surroundings
-Insulate with polystyrene cup
-Bomb calorimeter
Required practical 2a
Other than heat loss to surroundings, how else might this practical be improved?
-Read thermometer at eye level to reduce parallax errors
-Stir
-Use a digital thermometer for more accurate and fast readings
-Use greater masses and concentrations leading to a greater temperature change and therefor smaller percentage uncertainty
Required practical 5
Butanone can be made by oxidising butan-2-ol with a mixture of sodium dichromate (VI) and sulfuric acid. Describe how you would then remove the butane from the reaction mixture
-Distillation
-Butanone has a lower BP than butan-2-ol (no hydrogen bonds)
-Transfer reaction to a round bottom flask with side arm
-Add anti-bumping granules (creates smaller bubbles so whole mixture doesn't go into condenser)
-Clamp round-bottomed flask over electric heater
-Add bung with integrated thermometer
-Attach liberg condenser to side arm
-Apply heat using condenser
-Collect distillate when thermometer shows bp of butanone
Preparation of ethanal by the oxidation of ethanol
-Make the oxidising agent by dissolving potassium dichromate (VI) in dilute sulfuric acid
-Using a 25 cm3 measuring cylinder, carefully measure out 12 cm3 of the acidified potassium dichromate(VI) solution and pour this into a boiling tube
-Cool the boiling tube in a beaker of cold water.
-Measure 2cm of ethanol and add with pipette to oxidising agent
Difference between distillation and reflux
Distillation = Heat and vapours are condensed and collected immediately , aldehyde
Reflux = the continuous heating of a sample where the vapours are condensed and returned to the vessel, aldehyde --> carboxylic acid
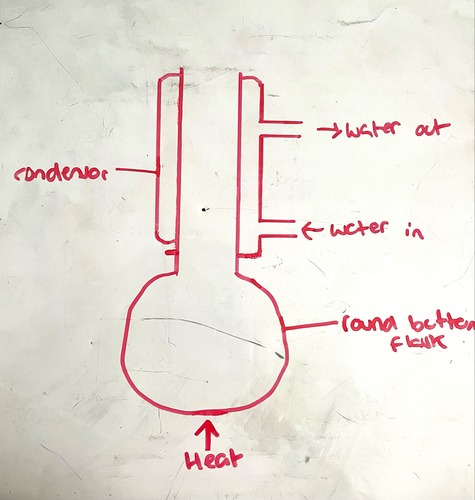
Reflux diagram
-Water in/out must be in correct order
-No horizontal lines covering openings/exits
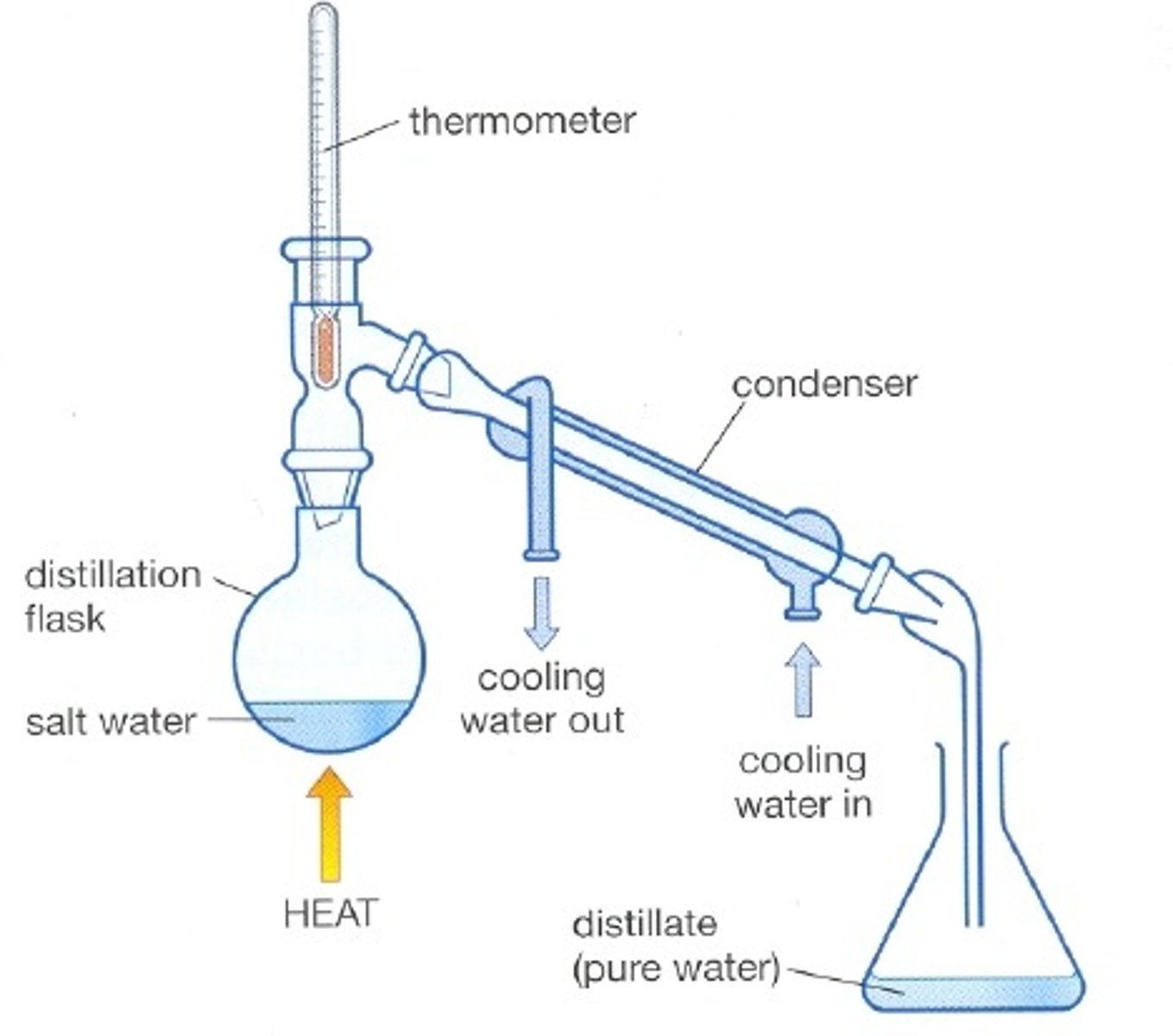
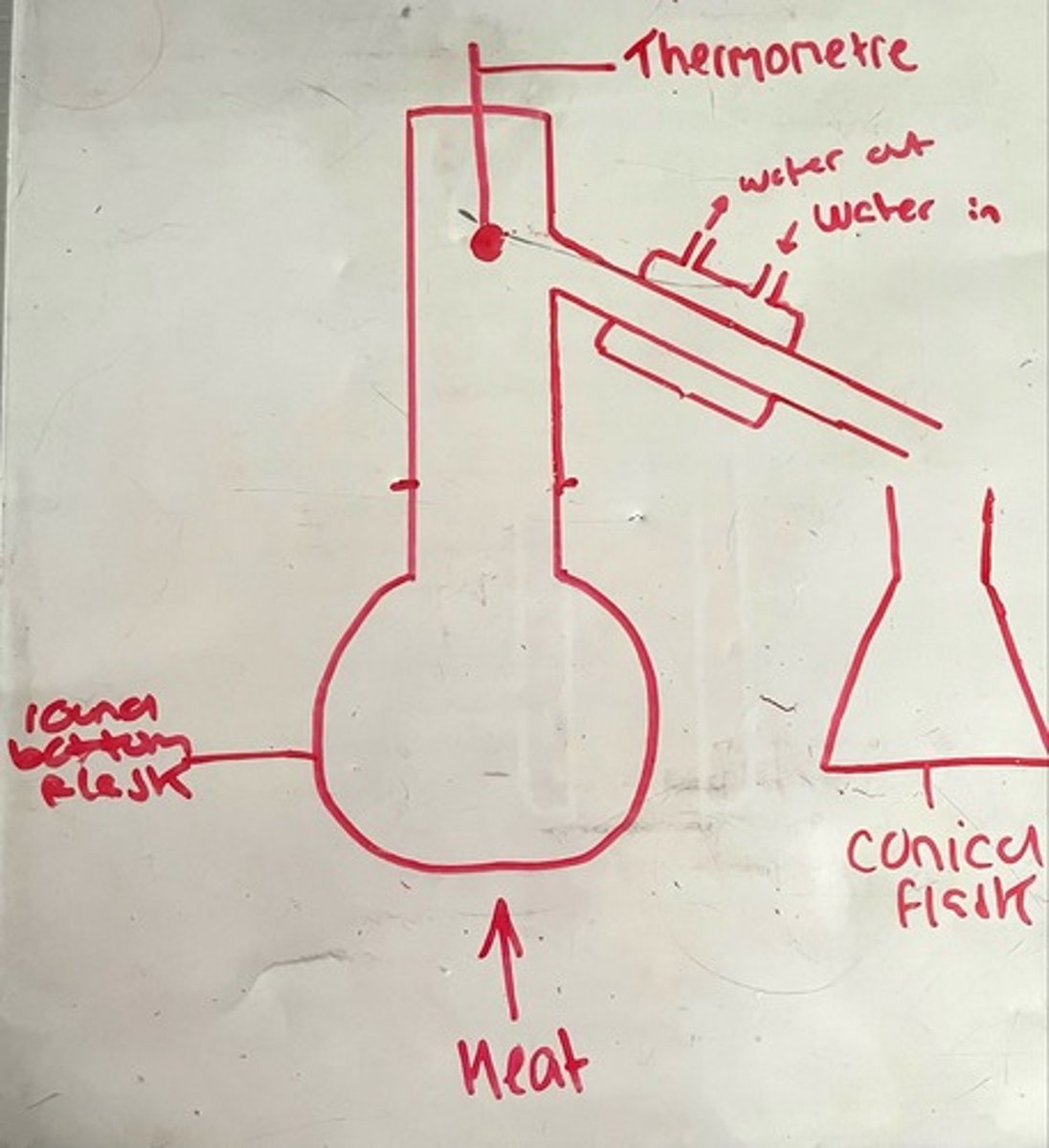
Distillation diagram
-No horizontal lines
-Thermometer must not go INTO the round bottom flask as it is measuring the temperatures of gasses leaving the flask
Heat source in distillation is water bath, as temperature of bunsen burner would be too difficult to control
Main differences between reflux and distillation apparatus
-Unlike reflux, in distillation the round bottom flask and condenser are not directly attached, they need an adapter
-Thermometer in distillation
Use of antibumbing granules
Prevents larger bubbles/form smaller bubbles
You are provided with a small sample of pure aspirin in a melting point tube. Describe briefly how you would determine an accurate value for the melting point of aspirin
Heat melting point tube in an oil bath, slowly near the melting point
RP5
a) State the chemical change that causes the solution in the flask to appear green at the end of the reaction of the oxidation of a primary alcohol
b) Why is a water bath rather than a bunsen burner used
c) Suggest a reagent that could be used to confirm the presence of an aldehyde in the distillate. State the observation you would expect to make if an aldehyde were present.
a) Dichromate reduced/Cr3+ formed
b) Alcohol is flammable
c) Tollens reagent, silver mirror
Ethanol can be oxidised by acidified potassium dichromate(VI) to ethanoic acid in a two-step process.
ethanol ethanal ethanoic acid
In order to ensure that the oxidation to ethanoic acid is complete, the reaction is carried out under reflux.
Describe what happens when a reaction mixture is refluxed and why it is necessary, in this case, for complete oxidation to ethanoic acid.
A mixture of liquids is heated to boiling point for a prolonged time
Vapour is formed which escapes from the liquid mixture, is changed back into liquid and returned to the liquid mixture
Any ethanal and ethanol that initially evaporates can then be oxidised
Write a half-equation for the overall oxidation of ethanol into ethanoic acid
CH3CH2OH + H2O ---> CH3COOH + 4H+ + 4e-
Describe how a student could use chemical tests to confirm that a liquid
contained ethanal and did not contain ethanoic acid
Ethanal presence:
Add Fehlings solution and warm, red precipitate generated
Ethanoic absence:
Add sodium hydrogen carbonate, no effervescence observed = no acid present
Titration
A student identified use of the burette as the largest source of uncertainty in the experiment. Using the same apparatus, suggest how the procedure could be improved to reduce the percentage uncertainty in using the burette.
Justify your suggested improvement.
Use a larger mass of solid, so a larger titre value is recorded
Test for an alcohol: metal
-Add a small piece of metallic sodium to alcohol.
-Produces effervescence and white precipitate
-Dispose any excess sodium safely using the beaker of ethanol provided. (Sodium will completely react with the excess ethanol in order to be safely washed away because if any water comes into contact with the sodium there is a serious fire risk).
Test for an alcohol: potassium dichromate
-Add acidified potassium dichromate to the solution.
-Primary and secondary alcohols will reduced from orange dichromate(VI) ions to green chromium(III) ions.
It will remain orange if a tertiary alcohol is present
Test for an aldehyde: Fehling's solution
-In a test tube mix together equal volumes of Fehling's solution A and Fehling's solution B. The resultant Fehling's test reagent should be a clear dark blue solution.
-Add 5 drops of this test reagent to a test tube along with a few anti-bumping granules, then add the aldehyde.
-Warm gently for around two minutes in a beaker of hot water, gradually bring the beaker of water to boiling and maintain this
temperature for a few minutes.
-A brick red precipitate formed if an aldehyde is present.
Test for an alkene: bromine water
Add bromine water to alkene.
Shake the contents of the tube vigorously from side to side.
Bromine water decolourised from orange if an alkene is present.
Test for a carboxylic acid: sodium carbonate
Place spatula of solid sodium carbonate in a test tube and add
dilute ethanoic acid using a pipette.
Collect the gas produced and bubble through limewater (calcium hydroxide). It will turns cloudy if a carboxylic acid is present as CO2 is produced.
Test for a halogenoalkane: sodium hydroxide and silver nitrate
-Using a teat pipette, add 5 drops of 1-bromobutane to about sodium hydroxide solution in a test tube. (OH- ions replace the Br by nucleophilic substitution).
-Warm the contents of the test tube for a few minutes in a beaker filled with hot water at approximately 60°C.
-Acidify by adding dilute nitric acid and then add silver nitrate solution. (Nitric acid removes carbonate and hydroxide impurities which would form precipitates. Silver Bromide precipitate then forms).
In distillation, explain why the water should enter the condenser at the bottom and not at the top
Ensure condenser fills with water, ensuring more vapour condenses, condenser is cooler
Test for ammonium sulfate
Warm with NaOH, damp red litmus paper in the mouth of tube turns red (ammonium gas)
Add acidified BaCl2, white precipitate formed (sulfate)