Solubility_Rules
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
Soluble compounds
Li+, Na+, K+, Rb+, and Cs+ and ammonium NH4+
Cl-, Br-, and I- and C2H3O2-, HCO3-, NO3-, ClO3, SO4-

2
New cards
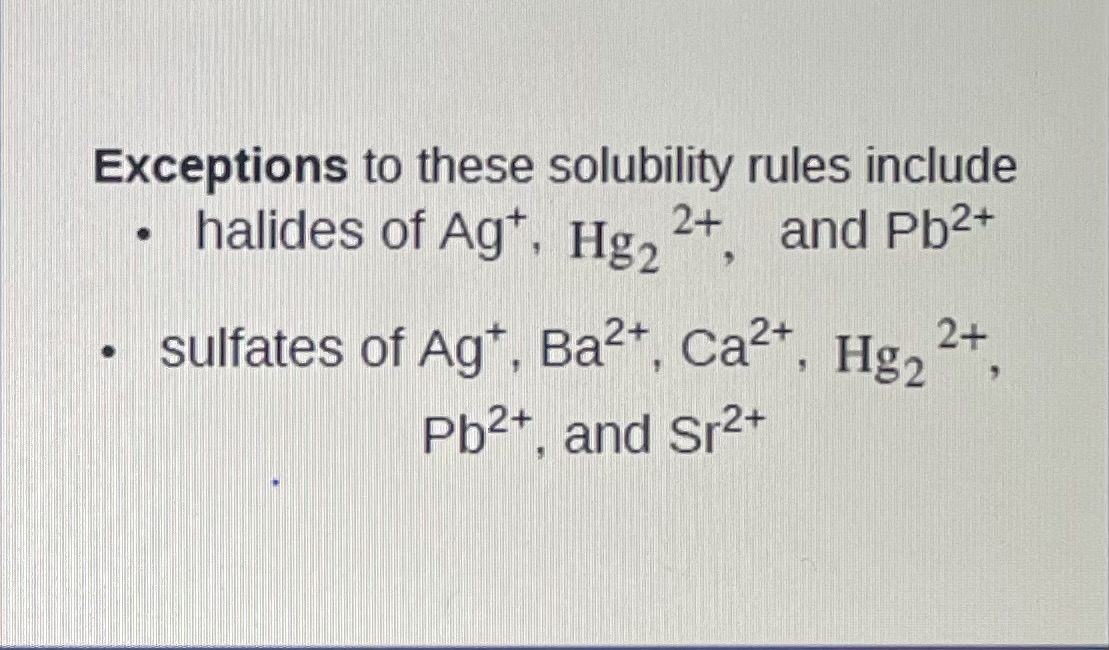
Exceptions for soluble
Ag+, Hg2^2+, Pb^2+ and Ag+,Ba^2+, Ca^2+, and Sr^2+
3
New cards
Insoluble compounds
CO3^2-, CrO4^2-, PO4^3-, S^2-, OH-

4
New cards
Exceptions for Insoluble compounds
compounds of these anions with group 1 metal cations and ammonium ion and hydroxides of group 1 metal cations and Ba^2+
