Chemical Reactions
0.0(0)
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
1
New cards
Reaction Rates are affected by:
-Temperature
-Concentration
-Surface Area
-Catalysts
-Inhibitors
2
New cards
Temperature
As temp increases, so does the movement of molecules, thus more collisions
3
New cards
Concentration
As concentration increases, so does the reaction rate. Reactants can collide with each other easily
4
New cards
Surface Area
As Surface Area increases, there is more “area” for substances to collide with
5
New cards
Catalyst
Enzyme that increases the rate of the chemical reaction without becoming part of the reaction
6
New cards
Inhibitors
Decreases the rate of a chemical reaction
7
New cards
Teddy
Collects
Sugar
Candies
Instantly
Temperature
Concentration
Surface Area
Catalysts
Inhibitors
8
New cards
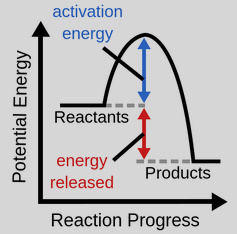
Exothermic Reaction
9
New cards
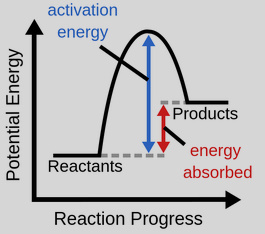
Endothermic Reaction