PT-630 Neurobiology Exam IV
1/126
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
127 Terms
THE BASAL GANGLIA ARE A COLLECTION OF SUBCORTICAL NUCLEI THAT CONTROL THE EXECUTION OF MOTOR PROGRAMS
BASAL GANGLIA
inputs
-striatum
-STN
outputs
-GPi
-SNr
interneurons
-GPe
modulator
-SNc (dopamine)
STRIATUM: INPUT (INHIBITORY)
The striatum is divided into three nuclei.
caudate and putamen
-separated by internal capsule
-dorsal striatum
-sensorimotor cortex input
nucleus accumbens
-ventral striatum
-reward/motivation
-limbic system input
spiny projection neurons (SPNs)
STRIATUM CELL TYPES
90–95% of striatal neurons
large, spiny dendritic arbors
GABAergic output cell
large aspiny neurons (LANs)
STRIATUM CELL TYPES
pacemaker at ~1–2 Hz
cholinergic interneurons
small & medium aspiny neurons
STRIATUM CELL TYPES
GABAergic interneurons
STN: INPUT (EXCITATORY)
subthalamic nucleus (STN)
input:
-frontal and motor cortices (glutamate)
-GPe (GABA)
output (glutamate):
-pacemake (~20 Hz)
-globus pallidus and substantia nigra pars reticulata.
GPI & SNR: OUTPUT
Globus pallidus internal segment (GPi) and substantia nigra pars reticulata (SNr) input:
-focal GABAergic input from SPNs.
-widespread excitatory input from the STN.
GPi/SNr output:
-pacemake (2–4 Hz)
-GABAergic projections to the thalamus.
-tonic suppression of movement.
GPE: MIDDLE MAN
Globus pallidus external segment (GPe) input:
-SPNs (GABA)
-STN (glutamate)
GPe output:
pacemake (~20 Hz)
-GABAergic projections to:
--striatum (feedback).
--STN (feedback).
--GPi/SNr (feedforward).
SNC: DOPAMINE
SNc neurons release dopamine (DA).
-Promotes movement.
-Plays neuromodulatory roles
in all basal ganglia nuclei.
-Best studied in the striatum.
DA neurons die during normal aging.
-large terminal fields
-pacemake with Ca2+
->50% of DA neurons must die in order to observe any behavioral phenotypes.
THE HYPERDIRECT PATHWAY SUPPRESSES MOVEMENTS
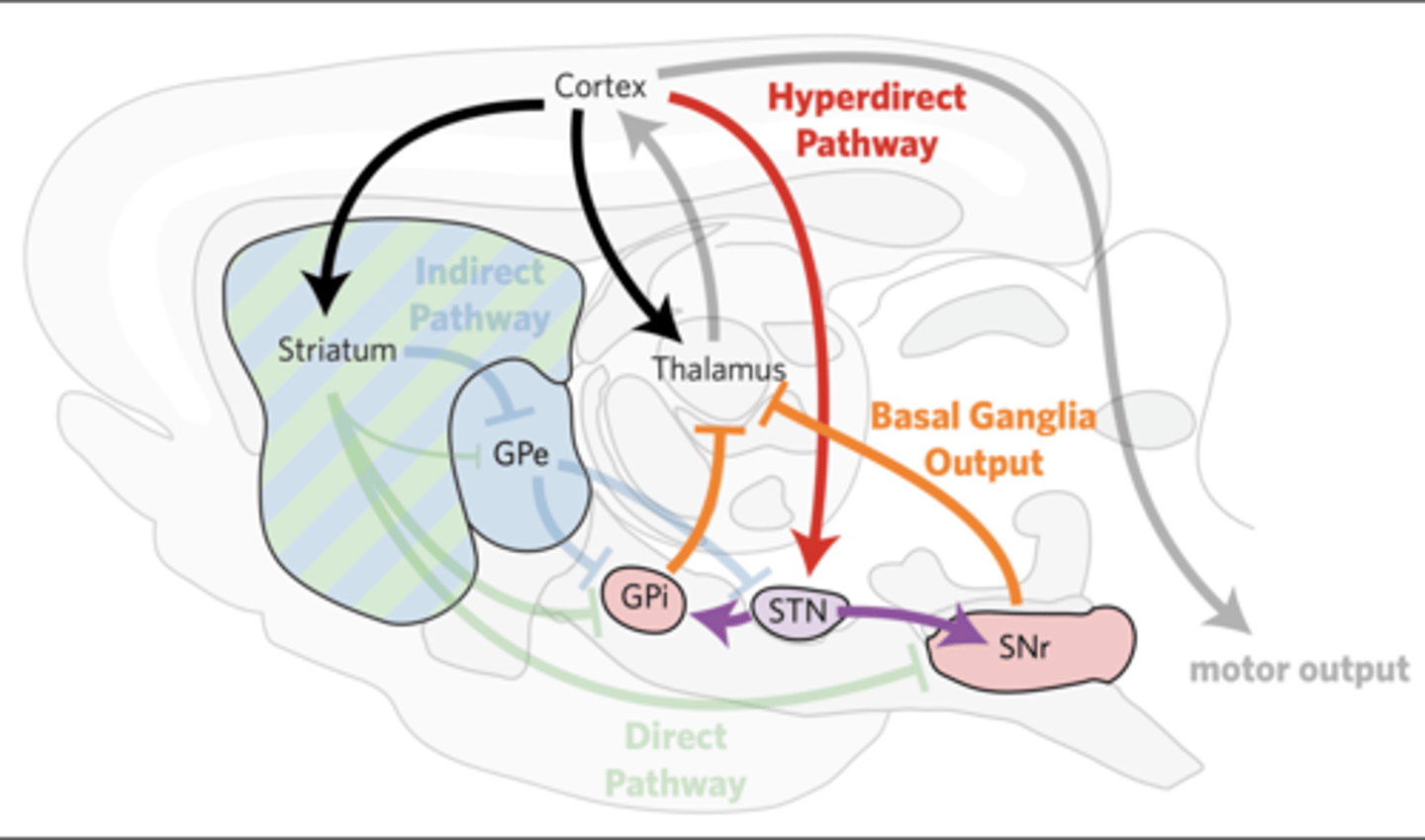
In the hyperdirect pathway, the cortex excites the STN, which directly excites the GPi/SNr within 5–8 ms.
THE HYPERDIRECT PATHWAY SUPPRESSES MOVEMENTS
Global increase in basal ganglia output.
-generally suppresses movement
-greater contrast for direct pathway input
Lesions in the hyperdirect pathway lead to hemiballismus
THE DIRECT PATHWAY SUPPRESSES MOVEMENTS
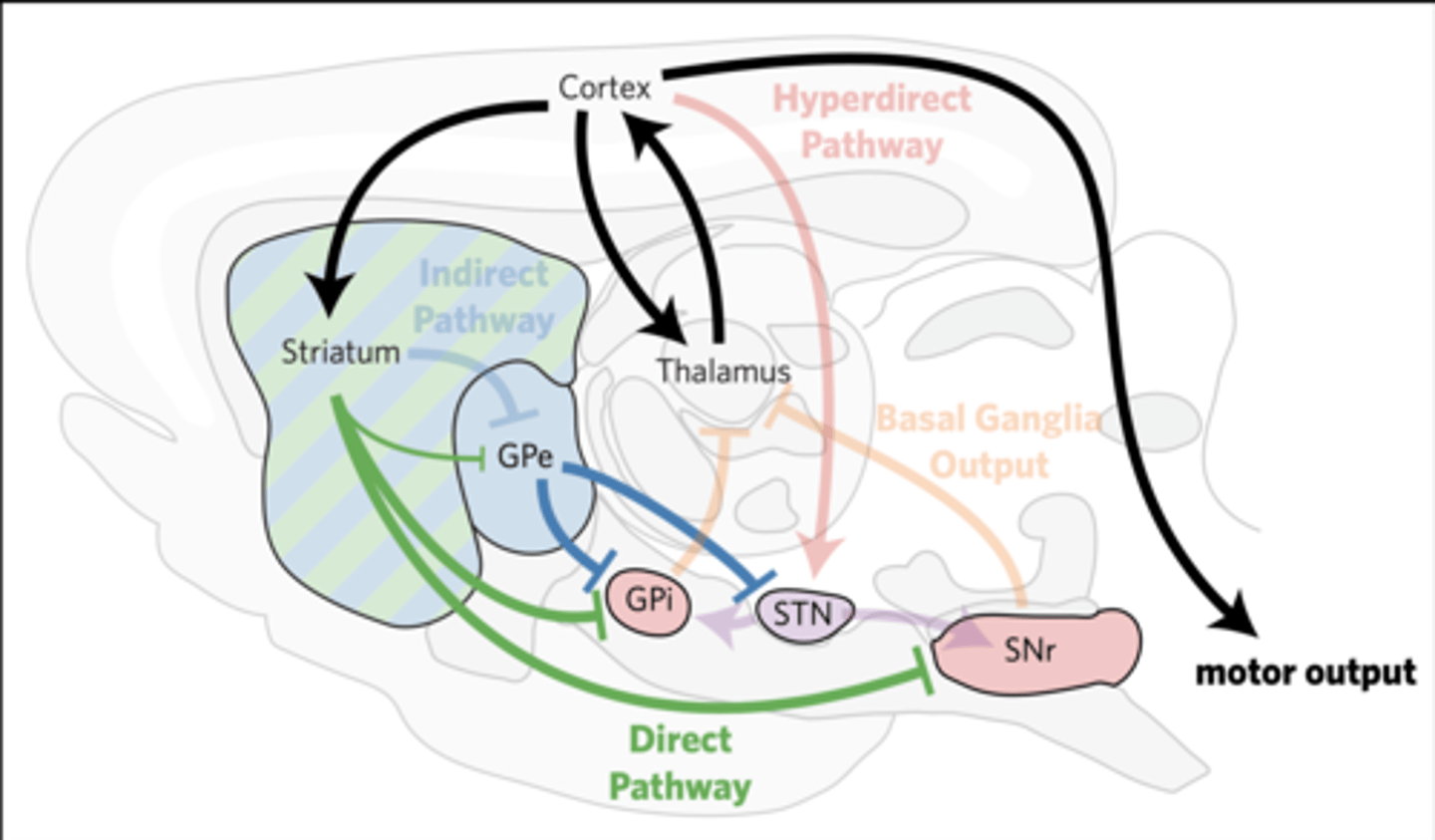
THE DIRECT PATHWAY PROMOTES MOVEMENT
dSPNs directly inhibit the GPi/ SNr within 15–20 ms, relieving their tonic inhibition of the thalamus and midbrain.
Thalamic activity promotes movements
(the direct pathway is called the “go” pathway).
Decreased activation of the direct pathway slows and prevents voluntary movements (termed bradykinesia and hypokinesia).
THE INDIRECT PATHWAY INHIBITS MOVEMENT
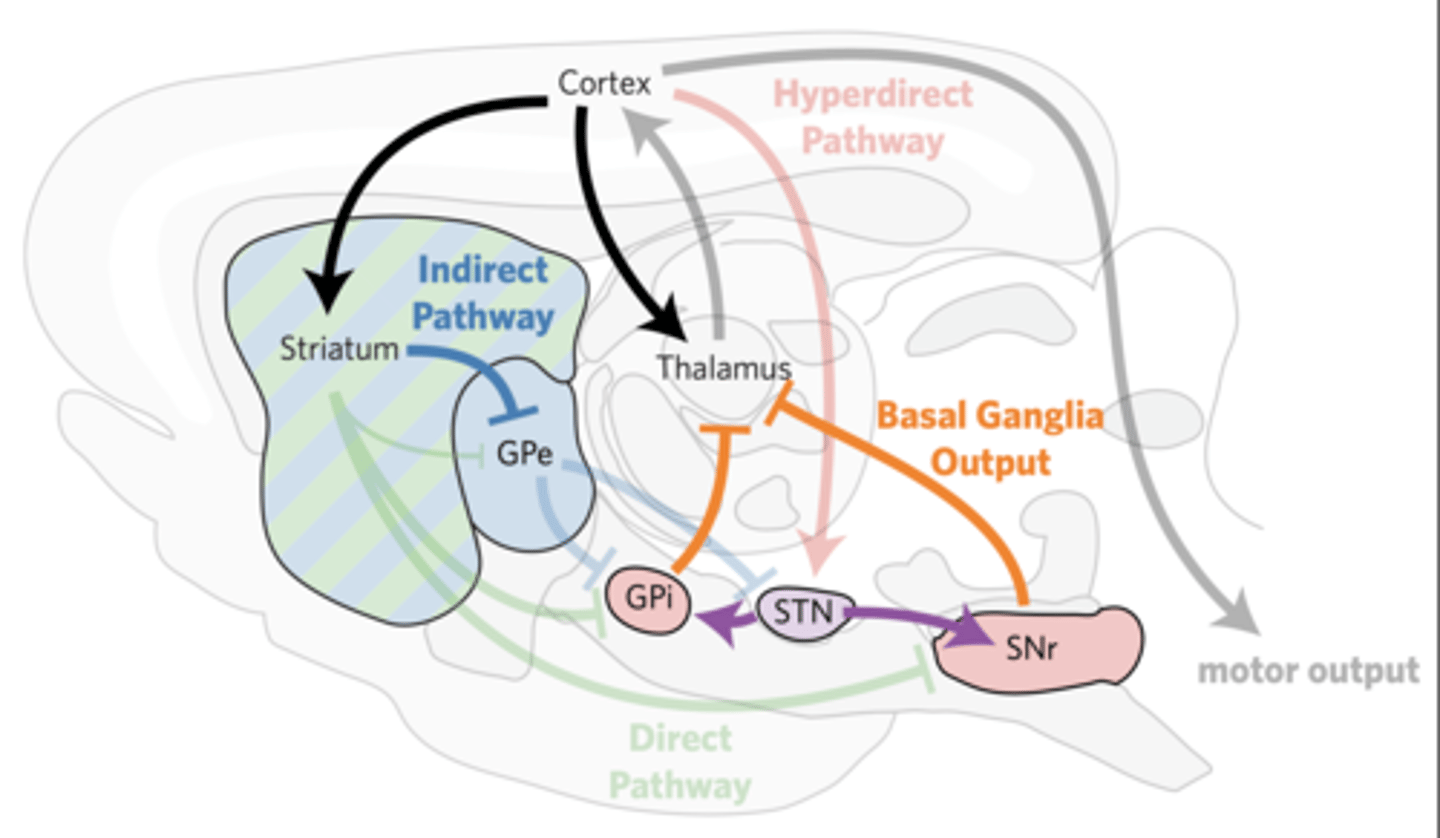
iSPNs indirectly excite the GPi/SNr within 30 ms, suppressing thalamic activity.
THE INDIRECT PATHWAY INHIBITS MOVEMENT
iSPNs inhibit GPe.
GPe disinhibits STN and GPi/
SNr.
STN neurons excite GPi/SNr.
Reducing thalamic activity curbs movement
(the indirect pathway is called the “no go” pathway).
Selective loss of iSPNs leads to persistent, uncontrolled jerky movements (termed chorea).
SPNS IN THE DIRECT (DSPNS) AND INDIRECT PATHWAYS (ISPNS) ARE GENETICALLY DISTINCT
Both dSPNs and iSPNs are GABAergic.
dSPNs express dynorphin, substance P, and D1 dopamine receptors.
iSPNs express enkephalin and D2 dopamine receptors.
D1 dopamine receptors (D1Rs & D5Rs) are Gs GPCRs that excite neurons.
DA RECEPTORS DIFFERENTIALLY REGULATE NEURONAL EXCITABILITY
Gs increases cAMP by activating adenylate cyclase.
cAMP depolarizes the cell via cyclic nucleotide gated (CNG) cation channels.
D2 dopamine receptors (D2Rs, D3Rs & D4Rs) are Gi/o GPCRs that depress neurons.
DA RECEPTORS DIFFERENTIALLY REGULATE NEURONAL EXCITABILITY
Gi/o decreases cAMP by inhibiting adenylate cyclase.
Reduced cAMP levels hyperpolarizes the cell by inhibiting CNG channels.
THE ACTIVITY OF LANS SHIFTS STRIATAL OUTPUT TOWARD THE INDIRECT PATHWAY
dSPNs express M4 receptors (Gi) and are inhibited by ACh.
iSPNs express M1 receptors (Gq) and are activated by ACh.
LAN activity decreases during initiated movements.
-inhibited by DA (D2)
-facilitates direct pathway to complete movements
THE BASAL GANGLIA MAY SELECT SPECIFIC MOTOR PROGRAMS AND INHIBIT COMPETITORS
Within 5–8 ms, the hyperdirect pathway activates the GPi/SNr, which provides general inhibition of motor programs and turns off any potentially competing motor programs.
Within 15–20 ms, the direct pathway inhibits specific GPi/SNr neurons to indirectly activate a desired motor program.
Within 30 ms, the indirect pathway indirectly activates the inhibited GPi/ SNr neurons to cease the execution of the desired motor program.
The basal ganglia promote the swift motor program execution
BASAL GANGLIA FUNCTION IS ILLUSTRATED BY LESIONS
muscimol (GABA agonist) decreases striatal output
contralateral bradykinesia
unaffected movement initiation
The basal ganglia inhibit inappropriate motor programs
BASAL GANGLIA FUNCTION IS ILLUSTRATED BY LESIONS
IEM-1460 (GluA2-free AMPAR antagonist) increases striatal output by inhibiting interneurons
contralateral dyskinesia
ANTIPSYCHOTICS ALTER BASAL GANGLIA FUNCTION
Many antipsychotics are D2 antagonists.
dystonia
acute reaction: increased BG output
abnormal distorted face & limb position
tardive dyskinesia
long-term use: decreased BG output
uncontrolled mouth movements
IN THE RETINA, LIGHT SENSED BY PHOTORECEPTORS IS PROCESSED BY CELLS IN THE INNER NUCLEAR LAYER BEFORE BEING SENT TO RETINAL GANGLION CELLS
Light initially passes through the cornea
LIGHT IS FOCUSED ONTO THE RETINA THROUGH THE VITREOUS FLUID
The iris is a muscular aperture that opens and closes to adjust the amount of light that enters our eyes.
The lens is a lens-shaped meshwork of the crystallin proteins.
Ciliary muscles contract to adjust the shape of the lens.
This changes the plane of focus for light, allowing us to see closer or farther distances
outer nuclear layer: photoreceptors
THE RETINA CONTAINS THREE LAYERS WITH SIX MAIN CLASSES OF CELLS
rods: dim light
cones: wavelength-specific
inner nuclear layer: interneurons
THE RETINA CONTAINS THREE LAYERS WITH SIX MAIN CLASSES OF CELLS
bipolar cells: glutamatergic input to
amacrine and ganglion cells
horizontal cells: GABAergic feedback to photoreceptors
amacrine cells: deliver a variety of different inputs onto ganglion cells
ganglion cell layer: projection neurons
THE RETINA CONTAINS THREE LAYERS WITH SIX MAIN CLASSES OF CELLS
retinal ganglion cells (RGCs): project to midbrain, hypothalamus, and thalamus (LGN)
The axons from ganglion cells have to pierce through the retina (at the optic disc) in order to exit through the back of the eye and migrate toward the LGN.
photopigments
retinal: small molecule that absorbs
370 nm light
opsin: GPCR
Δ wavelength sensitivity
creates intracellular signal
RODS AND CONES EXPRESS PHOTOPIGMENTS, WHICH ABSORB LIGHT
Cones express color-sensitive opsins:
L-opsins: red/yellow
M-opsins: green/yellow
S-opsins: violet/blue
Rods express a relatively color-insensitive opsin called rhodopsin
Opsin activates the G protein transducin
activates phosphodiesterase •
hydrolyzes cGMP
cyclic nucleotide-gated cation channel
↓cGMP,↓pNa,↓Vm
reduced glutamate release
fovea: high acuity & color
corresponds to center of our
visual field
highest concentration of cones
1:1 mapping to RGCs
foveola: greatest acuity
only outer nuclear layer
midget cones
periphery: high sensitivity
mostly rods
15-30:1 mapping to RGCs
off bipolar cells
BIPOLAR CELLS CAN BE HYPERPOLARIZED OR DEPOLARIZED BY LIGHT
iGluRs (kainate and AMPA type)
hyperpolarized by loss of glutamate
only used with cones
(color-opponent processing)
on bipolar cells
BIPOLAR CELLS CAN BE HYPERPOLARIZED OR DEPOLARIZED BY LIGHT
mGluRs (Gi)
depolarized by the loss of glutamate
used with rods & cones
Bipolar cells release glutamate onto amacrine and ganglion cells.
Light hyperpolarizes photoreceptors, reducing their excitatory input onto horizontal cells.
As a result, horizontal cells are less active, reducing GABA release to nearby photoreceptors.
Nearby photoreceptors that are not activated by light will thus be depolarized, increasing their amount of glutamate release.
This increases the contrast at borders of light and dark.
In center-surround organization, the center and periphery of the RGCs receptive field respond differently to light.
on-center RGCs: activated by light in the center; inhibited by light in the periphery
off-center RGCs: inhibited by light in the center; activated by light in the periphery
Center-surround is created by horizontal cells
THE OPTIC NERVES AND TRACTS PROJECT FROM THE RETINA TO THE LGN
optic nerve: myelinated RGC axons
optic chiasm: convergence
optic tracts:
-medial root:
--superior colliculus
--pretectal nucleus
--reticular formation
-lateral root: LGN
M cells are RGCs that project to the magnocellular layers (1–2).
THE LGN SEPARATES CHROMATIC & ACHROMATIC CELLS
fast AP conduction
rapid desensitization
responsive to dim light
(large dendritic arbors)
P cells are RGCs that project to the parvocellular layers (3–6)
THE LGN SEPARATES CHROMATIC & ACHROMATIC CELLS
smaller receptive fields
sensitive to red & green light
K cells are RGCs that project between layers and respond to blue light.
THE LGN SEPARATES CHROMATIC & ACHROMATIC CELLS
larger receptive fields
only center, no surround
OPTIC RADIATIONS
PROJECT FROM LGN TO V1
primary visual cortex (V1)
-calcarine sulcus
-ocular dominance columns
-retinotopic map
-respond to very specific visual stimuli
Higher-level information is extracted in visual association cortices.
RGCs, LGN, & V1: color-opponent cells
COLOR VISION IS BUILT AT MULTIPLE LEVEL
“Red” neurons are excited by L- cones inhibited by M-cones.
“Green” neurons are excited by M-cones and inhibited by L-cones.
“Yellow” neurons are excited by L- and M-cones and inhibited by S- cones.
“Blue” neurons are excited by S- cones and inhibited by L- and M- cones.
Light in center = increase in RGC's
Light in surround = decrease in RGC's
All Primary Visual Cortex sees is lines & angles
Shaped & colors are determined in higher structures later on
Chorea
“dance”
continuous
fluid or jerky
Ballismus
large amplitude
flinging
involuntary movements: chorea + ballismus potential sites
iSPNs & STN
GPi, SNr
decreased movement
brady- = slowed
hypo- = decreased
a- = absence
brady-, hypo-, and akinesia: potential sites
SNc (DA)
dStr (dSPNs)
GPe
rigidity: potential sites
dStr
SNr (head)
GPi (body)
Tremor
resting tremor
-decreases during movement
-seen in PD
-due to oscillations in basal ganglia?
intention tremor
-present during targeting
-cerebellar sign
Rigidity
resistance to passive movement
clasp-knife = CST
lead pipe = basal ganglia
cogwheel = lead pipe + tremor
Parkinsonian signs
resting tremor
bradykinesia
cogwheel rigidity
unsteady gait
Parkinson’s disease is caused by
SNc degeneration and improves with levodopa.
Drug-induced parkinsonism is caused by
D2 antagonists
Long-term use of antipsychotics leads to tardive dyskinesia.
Multisystem atrophy
caused by marked basal ganglia degeneration,
does not improve with levodopa,
Progressive supranuclear palsy
includes vertical gaze palsy.
Dementia with Lewy bodies
is a form of dementia that includes parkinsonian signs.
HD is caused by decrease in ISPN's (Dorsal Striatum)
4 nuclei of thalamus
Non-specific
Motor
Sensory
-association
Cognitive
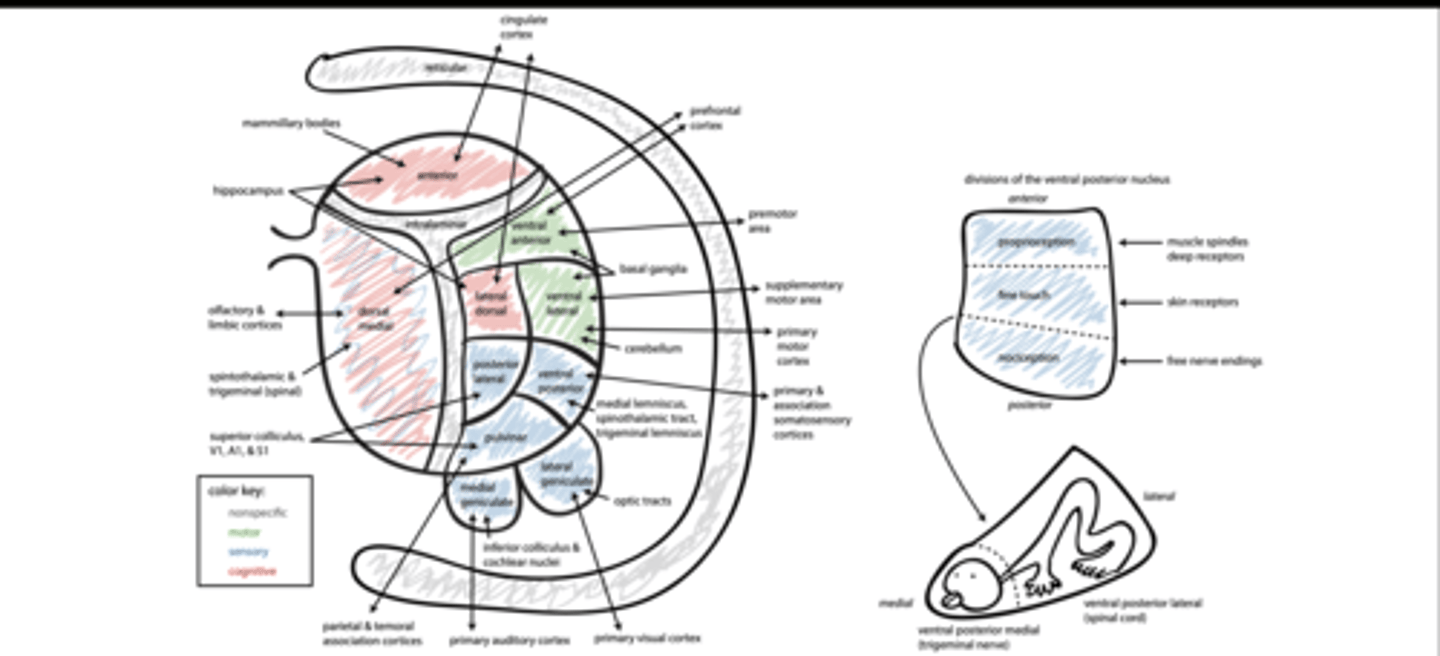
projection neurons
THE THALAMUS AND CORTEX HAVE RECIPROCAL CONNECTIONS
core: layer 4, focal
matrix: layer 1, diffuse
thalamic peduncles
THE THALAMUS AND CORTEX HAVE RECIPROCAL CONNECTIONS
anterior: PFC & cingulate
posterior: occipital & parietotemporal
superior: premotor, motor, and somatosensory
inferior: anterior temporal & orbital
Motor nuclei of thalamus
VAN: motor planning
VLN: motor output
Sensory nuclei of thalamus
VPN: somatosensation
LGN: vision
MGN: audition
PL/PuN: multimodal
association
DMN: olfaction & pain
THE INTRALAMINAR NUCLEI HAVE DIFFUSE CORTICAL PROJECTIONS
subcortical inputs:
-spinothalamic tract
-ascending arousal system
outputs:
-cortex (arousal)
-striatum (action)
function:
-synchronize activity
-facilitates communication
THE THALAMIC RETICULAR NUCLEUS IS AN INHIBITORY FILTER
input:
-all thalamocortical and
-corticothalamic fibers
ascending arousal system
-α2ARs
-M2 AChRs
TRN neurons (gap junctions)
output (GABA):
-other thalamic nuclei
function:
-noise reduction
-selective attention
TRN NEURONS FILTER THALAMIC ACTIVITY
filter out noise (weak activity)
-thalamic nuclei stimulate TRN
-diffuse GABAergic feedback
select which thalamic neurons impact cortical activity
-visTRN target LGN
-less active when attending to
that sensory modality
-more active when attending to other sensory modalities
sleep spindles
TRN NEURONS CONTRIBUTE TO SLEEP
bursts of TRN activity
observed during stage 2
function unclear (memory?)
inhibits sensory nuclei
TRN NEURONS CONTRIBUTE TO SLEEP
Spindles only occur in TRN neurons that target sensory nuclei.
TRN neurons that target limbic nuclei don’t exhibit spindles.
THALAMIC DAMAGE
loss of function
-damaged relay neurons
synesthesia
-loss of thalamic output to TRN (i.e., disinhibition)
-strengthening of weak synapses
thalamic pain
-present in ~25% of people with thalamic strokes
-mechanism may be similar to synesthesia
THE OLFACTORY SYSTEM PROVIDES DIRECT INPUT TO THE LIMBIC CORTEX
nasal cavity
lined with respiratory (not sensory) and olfactory epithelia
olfactory epithelium
contains olfactory sensory neurons
olfactory nerve
formed by olfactory sensory neurons (1° afferents)
olfactory bulbs
connects olfactory nerve to olfactory tracts (2° afferents)
OLFACTORY SENSORY NEURONS (OSNS)
project olfactory cilia (or hairs) into nasal cavity
-increases surface area
-express one type of
-odorant receptor
form CN1
-axons pierce cribriform plate
-synapse in olfactory bulbs
replaced every 1–12 months
-basal cell = stem cell
-air damages plasmalemma
Odorant Receptors
GPCRs (Gs)
-bind one or a few odorants
cAMP-gated cation channels
-depolarize OSNs
glomeruli
OLFACTORY BULBS
collections of OSN axons & mitral cell dendrites
correspond to one specific odorant receptor
lateral inhibition
OLFACTORY BULBS
periglomerular cells stimulated by OSNs
granule cells stimulated by mitral cells and feedback from piriform cortex
OLFACTION PATHWAYS
2° afferents project to piriform lobe (connected to limbic system)
-amygdala(emotion)
-entorhinal cortex (memory)
3° afferents project to:
orbitofrontal cortex (conscious perception)
-hippocampus(memory)
-hypothalamus(autonomic
response)
-olfactory bulbs (modify sensitivity via granule cells
ENTORHINAL CORTEX
located in anterior PHG
main input/output of the hippocampus
reciprocal connections with:
-association cortices
-hippocampal complex
memory functions
-encoding: input to hippocampus alters circuits
-consolidation: output to cortex alters circuits
HIPPOCAMPAL STRUCTURE
Hippocampal structure
allocortex (3 layers)
medial temporal lobes
three general areas:
-dentate gyrus (DG): input
-hippocampus(CA1–4): relay
-subiculum: output
general circuit:
-DG→CA→sub
entorhinal cortex
Hippocampal connections
-perforant path (DG)
-alvear path (CA3 & CA1)
-subiculum→EC
Papez Circuit
Hippocampal connections
-linked to other limbic
structures by fornix
-involved in memory function
Hebbian plasticity
LONG-TERM POTENTIATION
fire together, wire together
Co-activation of pre- and postsynaptic neurons strengthens synapses
long-term potentiation (LTP)
NMDA receptors
-Mg2+ block (voltage)
-glutamate (ligand)
Ca2+ influx stimulates:
-CaMKII, PKA, & CREB
-↑AMPAR conductance & abundance
POTENTIATING LTP
fast excitatory input synchronizes
neuron activity
-amygdala
-septum
neuromodulators provided via fornix
-ACh (septum): M1AChRs (Gq)
-NE (LC): βARs (Gs) N N O
-5-HT (raphe): 5-HT4 (Gs) cAMP ATP
-DA (VTA): D1Rs (Gs)
HIPPOCAMPAL LESIONS PRODUCE PROFOUND AMNESIA
patient HM
-epileptic
-bilateral medial
-temporal lobe removal
-complete anterograde amnesia
THE ANTERIOR CINGULATE CORTEX HAS DIVERSE FUNCTIONS
emotional area
-active during positive emotions
-silent during negative emotions
other areas:
-motor planning
-pain
-bladder
-vocalization
-autonomic
THE AMYGDALA DETERMINES EMOTIONAL SALIENCE
limbic areas:
hippocampus: links emotion & memory
insula: links pain & emotion
nucleus accumbens: links positive & negative emotion
association cortices (perceptions & memories)
autonomic responses:
arousal (locus coeruleus)
heart rate (medulla)
stress (hypothalamus)
THE AMYGDALA RECOGNIZES AND PRODUCES FEAR & ANGER
activated when viewing fearful faces
damage produces fearlessness
-patient SM: bilateral damage
animal studies
hyperactivity is associated with anxiety