THIOL & ETHER
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
symmetrical ether.
If the alkyl substituents are identical,
the ether is a
unsymmetrical ether.
If the substituents are different,
the ether is a
Ether
less dense
less soluble in water
lower boiling points.
Ether
USE:
Solvents for fats
Oil
Waxes
Perfumes
Resins
Dyes
gums and hydrocarbons
are used
Insecticides
Miticides
fumigants for soil.
VAPORS OF CERTAIN ETHERS
biomolecular hydration
alcohol undergo into ether
diethyl ehter
DEHYDRATION is commertially to produce
Cleavage
Autoxidation
Reaction of ETHER
hydrobromic acid
Most ethers can be cleaved by
AUTOXIDATION
pontaneous oxidation of a compound in air.
oxygen
AUTOXIDATION - in the presence?,
ethers slowly autoxidize to FORM:
hydroperoxides
dialkyl peroxides.
hydroperoxides
dialkyl peroxides.
presence of oxygen, ethers slowly autoxidize to
FORM:
CLEAVAGE
what ether reaction is present?
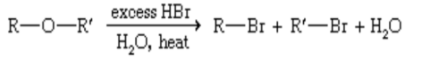
CLEAVAGE
what ether reaction is present?
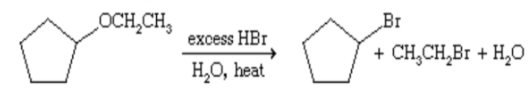
AUTOXIDATION
what ether reaction is present?
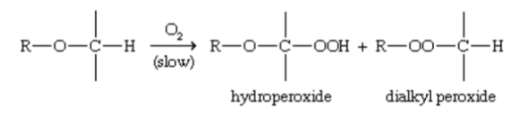
AUTOXIDATION
what ether reaction is present?
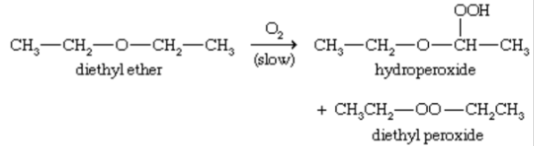
Thiol
sulfur analogs of alcohols.
mercaptans.
THIOL they are referred to as?
nature
Cysteine and Glutathione
A number of thiols are found in?
mercury
arsenic
THIOLS strong complexes with heavy metal cations such as WHAT ELEMENT?
Thioethers or Sulfides
analogues of ethers?
Thiol
unpleasant smell.
colorless oily liquids.
Thiol
insoluble in water but soluble in ethers & alcohols.
higher boiling point compared to those of corresponding ethers.
CIMETIDINE
used to prevent and treat symptoms of heartburn associated with acid indigestion and sour stomach.
CIMETIDINE
called H2 blockers.
It decreases the amount of acid made in the stomach.
ZOLLINGER-ELLISON SYNDROME
is a rare digestive disorder