Cell Cycle
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
Describe the stages of the cell cycle?
describe G1 phase?
1.cell cycle is G1; G stands for gap phase
2.Before a cell can divide, it needs to grow and roughly double in size during G1.
3.Throughout G1, the cell has a single copy of the genome. Toward the end of G1, the cell determines whether it is big enough and whether conditions are right for division
4.Once it decides to enter cell cycle: START point
what is the START or restriction point?
A point in the cell cycle, late in G1, when the cell makes the decision to divide. It is also called the restriction point of the cell cycle.
What is G0?
Instead of entering the cell cycle in late G1, a cell can decide to exit the cell cycle and differentiate. Cells that exit the cell cycle are said to be in G0. Some cells can remain in G0 for extended periods of time, even years, before reentering the cell cycle upon a signal.
What is the S phase?
cell needs to synthesize a second copy of the genome. At the end of S phase, the cell has two complete copies of the genome. After S phase, the cell enters a second gap phase called G2
What is G2?
During G2, the cell ascertains whether it is ready to divide. Most importantly, the cell takes time to ensure that a complete new copy of the genome has been synthesized without error.
What is M phase?
Finally, the cell enters M phase where mitosis occurs to equally segregate the two copies of the genome to the two daughter cells
what is flow cytometry
determine the proportion of cells in G1 or G2 phase. A powerful method to count and separate cells based on fluorescence intensity. Often used to separate cells based on their DNA content when the DNA is stained by a fluorescent dye
-Can determine the proportion of cells in G1 or G2 phase.
what is a cyclin?
What is a cyclin dependent kinase?
what are kinases and how do they function?
1.Kinases add a phosphate group to an OH side chain of a protein
2.A kinase only phosphorylates residues in the context of a certain set of neighboring amino acids, which gives specificity to kinases.
3.Phosphorylation of a protein can regulate its activity by changing its conformation or by modulating the binding of other proteins.
what are the different types of kinases?
1.Serine/threonine kinases phosphorylate the OH groups on specific S or T residues.
2. Tyrosine kinases phosphorylate OH groups on specific Y residues.
3.Dual specificity kinases can phosphorylate specific S, T, or Y residues
Not all S and T residues are targets of kinases
True, a kinase only phosphorylates residues in the context of a certain set of neighboring amino acids, which gives specificity to kinases
how can phosphorylation of a protein affect its activity?
by changing its conformation or by modulating the binding of other proteins
ex: CDK1, used in mitosis with cyclinB. When CDK1 is bound to cyclin, but unphosphorylated, the T loop partially blocks the kinase activity of the CDK by blocking access to the ATP . Phosphorylation of CDK1 at Thr 161 causes a conformational change, moving the T loop with Thr 161 away from the ATP, making the kinase active and the ATP site is now open
what are phosphatases?
1.Phosphatases work in opposition to kinases by removing phosphates from proteins
2.Therefore proteins can be regulated by a balance of kinases and phosphatases. When the kinase wins, the protein is phosphorylated and in a certain state of activity. When the phosphatase wins, the protein is not phosphorylated and the protein is in the opposite state of activity.
how are proteins targeted for degradation?
1.covalent modification that attaches a short polypeptide, ubiquitin, to the protein. A short polypeptide that can be covalently attached to a lysine on a target protein. When a chain of these peptides are added to a protein, the protein is targeted for degradation
what are ubiquitin ligase enzymes?
A class of proteins that specifically attach ubiquitin to lysine residues in their target proteins.
what is a proteasome?
1.A large, 26S, cylinder-shaped protein complex that degrades proteins.
2.The poly-ubiquinated protein is then targeted to the 26S proteasome.
3.The proteasome can degrade unfolded proteins and poly-ubiquinated proteins.
4.The proteasome is used for both general protein turnover of old proteins and targeted degradation.
5.used for both general protein turnover of old proteins and targeted degradation
what is maturating-promoting factor (MPF)?
Maturation (or M phase)-promoting factor. An activity conserved across eukaryotes found in M-phase cells. If active MPF from any eukaryote is injected into an immature frog oocyte, it will induce maturation. MPF is not only active in meiosis but also active in mitosis and is sometimes referred to as mitosis-promoting factor
how does degradation of cyclin affect CDK?
1.At certain times in the cell cycle, the cyclin subunit of cyclin/CDK is poly-ubiquinated by a ubiquitin ligase and then degraded at the proteasome.
2.Degradation of cyclin inactivates the CDK, which is not active without its partner cyclin.
what are cyclin/CDK complexes and their function?
Different combinations of cyclins and CDKs regulate key steps of an integrated cell cycle.
Each different combination of cyclin/CDK as a domino in the cell cycle. Each cyclin is made during parts of the cell cycle and is then degraded at a specific step so the cell cycle cannot go backward.
Also remember that CDKs are drawn from a constant pool. Each step is regulated by a series of kinases, phosphatases, and ubiquitin ligases
1.cyclin D
2.cyclin E
3.cyclin A
4.cyclin B
describe cyclin D/CDK
this is the cyclin responsible for moving the cell past START. Cyclin D/CDK targets discussed include Rb. Active at the end of G1 and regulates the entry into the cell cycle.
describe cyclin E/CDK
regulates the initiation of S phase. CyclinE/CDK targets discussed include a DNA helicase, Cdc6, and centrosome components. Induces the duplication of the centrosome so that there are exactly two centrosomes in mitosis to ensure a bipolar spindle
describe cyclin A/CDK
The cyclin that is at high levels after the initiation of S phase until late G2. It is responsible for preventing the cell cycle from going backward or reinitiating S phase. CyclinA/CDK targets discussed include E2F and Cdc6.
Describe cyclin B/CDK
The cyclin responsible for initiating mitosis and anaphase onset. CyclinB/CDK targets discussed include Cdc20, Cdc25, Wee1, lamin, histone H1, condensin, and seperase.
what is E2F and what initiates S phase?
1.S phase, The phase of the cell cycle where DNA is synthesized, is a nonreversible step in the cell cycle, and is controlled by a central transcription factor E2F
2.When E2F is active, E2F induces the expression of genes encoding cyclinE and cyclinA. E2F binds to the promoters of the cyclinE and cyclinA genes to turn them on and cyclinE and cyclinA are made
what are cyclinE and cyclinA?
1.CyclinE responsible for initiating S phase. CyclinE/CDK targets discussed include a DNA helicase, Cdc6, and centrosome components.
2.CyclinA that is at high levels after the initiation of S phase until late G2. It is responsible for preventing the cell cycle from going backward or reinitiating S phase. CyclinA/CDK targets discussed include E2F and Cdc6
what inactivates E2F? What occurs in early G1 before S phase?
In early G1, a protein called Rb binds to and inactivates E2F, repressing (turning off) the cyclinE and cyclinA genes. Plays a central role in preventing cells from entering the cell cycle by inhibiting E2F. When phosphorylated by cyclinD, it can no longer bind E2F.
What inactivates Rb? What occurs in late G1 before S phase?
1.In late G1, the cell decides to enter the cell cycle by activating cyclinD/CDK activity. CyclinD/CDK phosphorylates Rb. Phosphorylated Rb can no longer bind or repress E2F.
2.So when cyclinD/CDK is active at START, E2F is activated, the cyclinE gene is turned on, and after a short delay, the cyclinA gene is activated
what happens when cyclinE is activated?
1.Once activated, cyclinE/CDK initiates S phase by phosphorylating multiple targets.
2.CyclinE/CDK phosphorylates a DNA helicase, which then begins to unwind DNA, allowing for access of DNA polymerase.
3.CyclinE/CDK also phosphorylates Cdc6 to fire the origins of replication. Once cyclinE/CDK has stimulated the firing of the origins of replication, DNA polymerase can progress to fully replicate DNA.
4.Finally cyclinE/CDK phosphorylates components of the centrosome to initiate its duplication in early S phase.
what are some of the mechanisms to ensure that the initiation of S phase is limited to exactly once per cell cycle (DNA replication)?
1.Regulate E2F
2.Regulate cdc6
how does the cell regulate E2F?
1.Throughout S phase and G2, cyclinA/CDK continuously phosphorylates E2F.
2.Phosphorylated E2F is unable to bind the cyclinE or cyclinA promoters, shutting off transcription
3.This ensures that cyclinE is only made at the G1 to S transition.
4.Importantly, cyclinE will not be made again later in the cell cycle, meaning that S phase cannot be reinitiated before mitosis.
how does the cell regulate cdc6?
1.In early G1, Cdc6 binds to the origins of replication.
2.Cdc6 then primes the origin of replication by recruiting other components to the origins, including helicases
3.As long as Cdc6 is present, the origin of replication cannot fire or initiate. So Cdc6 is a licensing factor for the firing of the origins of replication; the origin cannot fire without Cdc6 recruiting other components, but can also not fire until getting rid of Cdc6.
4.CyclinE/CDK phosphorylates Cdc6 at the initiation of S phase. Phosphorylated Cdc6 is then targeted to the proteasome
5.CyclinA/CDK continuously phosphorylates any Cdc6 in S or G2 phase, ensuring that no Cdc6 is around. Therefore Cdc6 cannot rerecruit components to the origins of replication and the origins cannot fire a second time in later S or G2 phase
Describe S phase
major process in S phase is duplication of the DNA. As DNA is replicated, the resulting two sister chromatids (each with one copy of DNA) must remain associated with each other until anaphase of mitosis to ensure that they segregate to opposite poles
1.A protein complex called cohesin forms a literal ring around the two sister chromatids
2.The Scc1 protein secures the opening of the ring. The cohesin ring is loaded onto chromatids in G1 and encircles both sister chromatids after DNA replication
3.The ring remains closed until anaphase, when the sister chromatids segregate apart from one another.
what is cohesin?
A complex of proteins that form a literal ring around sister chromatids, keeping them together from S phase until anaphase onset.
what is the Scc1 protein?
A protein that closes the cohesin ring. It is a target of the Seperase protease.
Describe the challenge the cell faces for activating cyclinB/CDK1, a key regulator of mitosis?
1.CDK1 remains at a constant level throughout the cell cycle but must remain inactive throughout most of the cell cycle and only become an active kinase at the onset of M phase when there is MPF activity
2.One major way to regulate CDK1 is that it must be associated with cyclinB to be active. The levels of cyclinB protein consistently rise throughout S and G2 phases
3.evels of cyclinB/CDK1 complexes also consistently rise in the cell cycle. Somehow, the cell must keep cyclinB/CDK1 inactive until the onset of mitosis, when all the cyclinB/CDK1 must be activated at once to drive cell into mitosis.
Describe the steps involved in regulating cyclin B/CDK activity
1.Starts early in S phase and continues through G2, cyclinB is being consistently made. As cyclinB is made, it binds to CDK1. Binding of cylinB causes a conformational change in CDK1 that exposes Thr161. Thr161 is then phosphorylated by a CDK activating kinase (CAK). Phosphorylation of Thr161 by CAK causes a further conformational change, which makes the CDK kinase activity 80–100 times more active
2.However, we do not want active CDK1 early in the cell cycle. CDK1 is quickly inactivated. The kinase Wee1 phosphorylates Tyr15 and Thr14 of CDK1 to make it inactive. Wee1 functions to inhibit MPF activity and is the brake for the cell cycle
3.steps 3 and 4, the cell is ready to enter mitosis and needs to rapidly activate all of its cyclinB/CDK1 complexes. Mitosis is initiated. Cdc25 is a phosphatase that removes the phosphates from Tyr15 and Thr14 of CDK1. So, Cdc25 activates CDK1 kinase (MPF) activity. Cdc25 is the accelerator of the cell cycle
4.speeds up Cdc25 activity and ensures that all of the cyclinB/CDK1 heterodimers are rapidly activated to ignite the bomb of MPF activity. Once Cdc25 activates a little bit of cyclinB/CDK1, the CDK1 kinase begins to have activity. Two targets of active CDK1 kinase are Wee1 and Cdc25. CDK1 phosphorylates and inhibits the activity of Wee1. Now that Wee1 is inactivated, it can no longer inhibit CDK1.
This negative feedback loop effectively puts the brakes on the cell cycle brake. Active CDK1 also phosphorylates and activates Cdc25. Now the hyperactive Cdc25 rapidly removes all the inhibitory phosphorylations on Tyr15 and Thr14 of CDK1. Furthermore, there is no active Wee1 to combat the activity of Cdc25.
In summary, how is CDK1 activity controlled?
a series of kinases and phosphatases precisely control CDK1 activity so MPF can suddenly burst to activity at the onset of mitosis but not earlier.
explain how cyclinB/CDK1 is inactivated to complete mitosis
CDK1 activity leads to the degradation of cyclinB. In effect, CDK1 activity turns itself off.
1.Active CDK1 phosphprylates its target Cdc20. Cdc20 is a subunit of the anaphase-promoting factor (APC).
2.Cdc20 and APC function together as an ubiquitin ligase to covalently attach multiple ubiquitins to lysine residues in cyclinB. Once ubiquitinated by Cdc20, cyclinB is targeted to the proteasome where it is degraded. The first 90 amino acids of cyclinB, containing multiple lysine residues that are targets of active Cdc20, is called the destruction box.
3.Once cyclinB is degraded, CDK1 is no longer active, MPF activity is turned off, and the cell can complete mitosis
4.The degradation of cyclinB prevents the cell cycle from going backward, as MPF activity cannot be turned on again until more cyclinB is made in the next cell cycle
what is the destruction box?
Found in the N-terminus of cyclin. It contains multiple lysines that are ubiquinated by Cdc20, leading to the degradation of cyclin.
What is a ubiquitin ligase?
A class of proteins that specifically attach ubiquitin to lysine residues in their target proteins.
What is cdc20?
A ubiquitin ligase. It is activated by cyclinB/CDK and adds ubiquitins to cyclinB and Securin.
What is APC?
The anaphase promotion factor. It works with Cdc20 or other ubiquitin ligases to add ubiquitin to its targets.
iHow is cyclinB/CDK kinase activity intiate mitosis?
1.In early M phase, more specifically prophase, cyclinB/CDK has multiple targets. One target is lamin (An intermediate filament in the nucleus that provides physical support to the nuclear envelope)
2.Phosphorylation of lamin by cyclinB/CDK leads to nuclear envelope breakdown, a key step of prophase that allows microtubules in the spindle to access kinetochores
3.CyclinB/CDK also phosphorylates histone H1 (The histone that interacts with DNA between nucleosomes) and condensin to initiate chromosome condensation
4.CyclinB/CDK also phosphorylates microtubule-associated proteins to change the behavior of microtubules and form the mitotic spindle
what are nucleosomes?
Complexes of histones that are the major protein component of chromatin.
why is it important for chromosomes to condense in mitosis?
In prophase, chromosomes must condense from an extended chromatin form in interphase to the condensed chromosomes seen in mitosis to avoid entanglement during segregation.
Condensin is related to cohesion and forms a similar ring around loops of a single sister chromatid that help in chromosome condensation
what is anaphase onset?
The moment in mitosis when all sister chromatids simultaneously detach from one another in order to segregate toward opposite spindle poles.
how does cyclinB/CDK activity lead to separation of sister chromatids at anaphase onset?
There are additional controls to ensure that all pairs of sister chromatids are aligned on the metaphase plate before anaphase onset
1.The target of cyclinB/CDK that induces anaphase onset is Cdc20. Cdc20 is an ubiquitin ligase that is a member of the anaphase promotion complex, or APC.
2.When Cdc20 is phosphorylated by cyclinB/CDK, it becomes active and begins to ubiquitinate its targets. The major target of Cdc20 to induce sister chromatid separation at anaphase onset is Securin (protein that binds to and inhibits Seperase.
3.CyclinB/CDK phosphorylates Cdc20, which then degrades Securin, releasing Seperase to cleave Scc1 and open the ring connecting sister chromatids. In this manner, all the sister chromatids are separated simultaneously at anaphase onset
what is seperase and scc1?
A phosphatase that targets Scc1 to open cohesin at anaphase onset. It is inhibited by Securin or cyclinB/CDK activity. Scc1 is the protein that closes the cohesion ring that connects sister chromatids
When Seperase cleaves Scc1, the cohesin ring opens, allowing sister chromatids to be pulled apart
what is securin?
A protein that binds and inhibits Seperase. It is degraded by Cdc20.
summarize regulatory events for CyclinB/CDK activity
1.CDK1 is in the nonphosphorylated form and is inactive
2.CyclinB is made, associates with CDK1
3.CAK phosphorylates CDK1 Thr161 and activates CDK1
4.Wee1 phosphorylates CDK1 Try15 and Thr14; CDK1 inactive
5.Cdc25 removes inhibitory phosphates; CDK1 is inactivated
6.CDK1 activates entry into mitosis
7.Cyclin B ubiquinated by Cdc20 and is degraded
8.Phosphatases (PP2A) remove phosphate from Thr161; CDK1 inactive
what two mechanisms exist to keep separase inactive?
1.Securin binds and inhibits separase activity
2.CyclinB/CDK phosphorylates separase to make it inactive
Why does the cell arrest in metaphase when high cyclinB/CDK activity induces Cdc20, which activates Seperase to open the cohesion rings?
There is an additional layer of control to regulate the irreversible step of anaphase onset to ensure that everything is ready.
1.First, when cyclinB/CDK levels are low, Securin binds and inactivates Seperase.
2.Another target of cyclinB/CDK is Seperase. When cyclinB/CDK phosphorylates Seperase, the protease is inactive, even if no Securin is around. When there is high cyclinB/CDK and Cdc20 activity, Securin is degraded, but Seperase is phosphorylated and inactive anyway
cyclinB/CDK needs to be activated to enter mitosis, and it also needs to be inactivated before the cell can complete mitosis
true, This is why when cyclinB levels are high, the cell remains in metaphase.
what are checkpoints and their function?
Quality control mechanisms that detect cellular damage and then act to pause the cell cycle to provide time to correct the problem.
has 3 components:
1.First, the checkpoint must somehow sense the abnormality in the cell (late DNA replication forks, DNA damage, or misaligned chromosomes at metaphase)
2.Signaling component that transmits the presence of an abnormality
3.An effector functions to inhibit the cell cycle machinery. By sensing the abnormality and transmitting that information directly to a molecule that can pause the cell cycle, checkpoints provide needed time for the cell to fix its problems.
What is Spindle Assembly or wait-anaphase checkpoint?
The spindle assembly checkpoint functions to ensure that anaphase onset does not proceed until all the chromosomes have been properly attached to the bipolar mitotic spindle. Provides time to the cell before anaphase onset
what is the sensor for the wait-anaphase checkpoint?
1.Sensor is an unoccupied kinetochore. Signaling components, including Mad2, localize specifically to unoccupied kinetochores. What is sensed by the kinetochore not attached to a microtubule is a lack of tension across the two kinetochores of the sister chromatids.
2.kinetochore functions as a tension meter. When every kinetochore is under bipolar tension, then anaphase is initiated. When all kinetochores are under bipolar tension, Seperase is activated and sister chromatids can be pulled toward opposite poles.
Describe the DNA damage checkpoint in G2
1.DNA damage checkpoint must be fast-acting because it does not have time to induce the transcription of new genes. In G2, DNA damage is recognized by a protein kinase called ATR
2.When ATR binds damaged DNA or a stalled replication fork, it phosphorylates a second kinase called Chk1
3.Chk1 kinase functions to delay the cell cycle by phosphorylating Cdc25 (protein phosphatase that removes inhibitory phosphorylations from CDK, activating cyclin/CDK activity)
4.Once Cdc25 is phosphorylated, it binds to an adaptor protein called 14-3-3 and is exported out of the nucleus and into the cytoplasm where it is inactive
5.Without Cdc25 activity, cyclinB/CDK remains inactive, giving time to the cell to repair its DNA damage before entering mitosis.
what is ATR?
ATM-related kinase. This kinase binds to damaged DNA in G2 and phosphorylates Chk1.
what is Chk1?
A kinase named for it mutant phenotype in yeast where the DNA damage checkpoint is defective. This kinase is activated by ATR and phosphorylates Cdc25.
what is cdc25
A protein phosphatase that removes inhibitory phosphorylations from CDK, activating cyclin/CDK activity. Mutations in this phosphatase inhibit cyclin/CDK activity and lead to large cells.
what is 14-3-3?
An adaptor protein that binds to and inactivates Cdc25 that was phosphorylated by Chk1.
Describe the DNA Damage Checkpoint in G1
In G1, the cell has more time to deal with DNA damage. The slow response DNA damage checkpoint functions by activating the transcription of genes that encode proteins that slow down the cell cycle
1.n G1, DNA damage is recognized by the ATM kinase (closely related to ATR)
2.ATM then phosphorylates and activates the Chk2 kinase (related to Chk1 kinase)
3.Active Chk2 kinase then phosphorylates and stabilizes the transcription factor p53.
What is ATM kinase?
Ataxia telangiectasia mutated kinase. This kinase binds to damaged DNA in G1 and phosphorylates Chk2.
What is Chk2?
A kinase named for it mutant phenotype in yeast where the DNA damage checkpoint is defective. This kinase is activated by ATM and phosphorylates p53.
what is p53 and its function?
A key player in the cellular response to DNA damage. This transcription factor turns on p21 to inhibit the cell cycle and pro-apoptotic factors to kill cells with too much DNA damage. This transcription factor is one of the cell’s best safeguards against cancer. More than half of all cancers have mutations in this tumor suppressor.
53 serves a key role as the activator of programmed cell death (apoptosis) in cells that take too long to repair DNA damage.
contrast the rapid response of G2 to the slow response of G1
1.For the rapid response to DNA damage, in G2, damage or stalled replication forks are recognized by ATR kinase, which phosphorylates Chk1. Active phosphorylated Chk1 phosphorylates Cdc25, which is then inactivated by 14-3-3.
2.For the slow response to DNA damage, in G1, DNA damage is recognized by ATM kinase, which phosphorylkates and activates Chk2. Chk2 phosphorylates and stabilizes p53. Unphosphorylated or MDM2-bound p53 is degraded. Active p53 is a transcription factor that turns on p21 to inactivate cyclin/CDK. p53 can also induce apoptosis
how does phosphorylation impact p53 function?
1.In normal conditions when there is no DNA damage, p53 is unstable and degrades. However, when DNA damage is present, and ATM and Chk2 kinases are active, p53 is phosphorylated by Chk2 to become stable
2.Once phosphorylated and stabilized, p53 functions as a transcription factor to turn on other genes. One of the genes activated by the stabilized p53 transcription factor is p21. The protein p21 is a CDK inhibitor.It physically binds to cyclin/CDK complexes to inhibit the kinase activity of the CDK
3.So, stable p53 activates p21 to slow down the cell cycle, giving time to repair the DNA damage before dividing.
Role of p53 and apoptosis?
Normally, in a cell with too much DNA damage, stabilized p53 increases the transcription of a number of pro-apoptotic factors. Once translated, these proteins go on to induce programmed cell death, protecting the organism from a potentially oncogenic mutation being propagated
what are some of the layers of regulation for p53?
1.regulation of p53 by Chk2 kinase
2.cond way to regulate and inhibit p53 is with the protein MDM2. MDM2 will bind to p53 and inhibit it by inducing the degradation of p53. Thus MDM2 acts in opposition to p53. As p53 is a tumor suppressor, MDM2 acts as an oncogene. A gain-in-function mutation activating one copy of the MDM2 gene will lead to overactive MDM2 and underactive p53. A lack of p53 will lead to cancer
what is wee1?
A protein kinase that phosphorylates and inhibits CDK. Mutations in this kinase lead to small calls that divide too early. Phosphorylates Tyr15 and Thr14 of CDK1 to make it inactive
What is Rb?
riginally found in families that inherited a high incidence of retinoblastoma and found mutated in nearly half of all cancers. It plays a central role in preventing cells from entering the cell cycle by inhibiting E2F. When phosphorylated by cyclinD, it can no longer bind E2F.
what is Mad1?
Localizes to kinetochores that are not attached to microtubules where it recruits and activates Mad2.
what is Mad2
Localizes to kinetochores that are not attached to microtubules and acts there to inhibit Cdc20
In order to understand anaphase onset more completely, you delete securin expecting premature sister chromatid separation. However, anaphase occurs at the proper time, not prematurely. You then do a screen for mutations that cause early sister chromatid separation in the securin deletion mutant background. You isolate a Serine to Alanine mutation in separase. You hypothesize that the point mutation in separase blocked __________, which normally prevents sister chromatid separation.
Phosphorylation by cyclinB/CDK
Ubiquitination by cdc20
Scc1 cleavage and opening of the cohesin ring
Phosphorylation by wee1
Phosphorlyation by cdc25
Phosphorylation by cyclinB/CDK
.CyclinB/CDK phosphorylates Cdc20, which then degrades Securin, releasing Seperase to cleave Scc1 and open the ring connecting sister chromatids. In this manner, all the sister chromatids are separated simultaneously at anaphase onset. So blocking phosphorylation will prevent sister chromatid separation
A null mutation in the S. pombe gene wee1 will lead to __________ yeast because __________
arger; cdc25 is inactivated
Smaller; T14 and Y15 remain unphosphorylated
Larger; T14 and Y15 are phosphorylated
Smaller; T161 is unphosphorylated
Smaller; T14 and Y15 are phosphorylated
Smaller; T14 and Y15 remain unphosphorylated
The kinase Wee1 phosphorylates Tyr15 and Thr14 of CDK1 to make it inactive
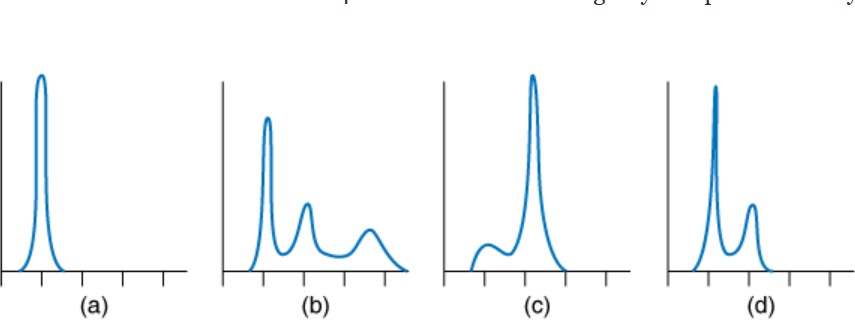
You treat normal cells with taxol for 24 h. Which of the following do you expect the flow cytometry to look like after the 24-h treatment with taxol?
C
taxol poisons cells by stabilizing microtubules. This prevents microtubules changing from their interphase configuration to a bipolar spindle. Without dynamic microtubules or a bipolar spindle, tension cannot be created across kinetochores. This would normally activate the spindle assembly checkpoint. Taxol treatment of normal cells leads to a mitotic arrest that can last hours.
Rb is an important component of the machinery to enter the cell cycle in late G1. Mutated Rb can lead to cancer. The mutated form of Rb that leads to cancer is __
A proto-oncogene
An oncogene
A tumor suppressor gene
A kinase
A phosphatase
tumor suppressor gene?
It plays a central role in preventing cells from entering the cell cycle by inhibiting E2F. When phosphorylated by cyclinD, it can no longer bind E2F.
Taxol is used as an anticancer agent. Its mechanism is to ________ microtubules and kill cells by _________.
Choose from the following options.
stabilize; allowing cancer cells to attempt mitosis
stabilize; arresting cells in mitosis
depolymerize; blocking mitosis
depolymerize; allowing them to bypass the spindle assembly checkpoint
increase the turnover of; arresting cells in mitosis
stabilize; allowing cancer cells to attempt mitosis
taxol stabilizing microtubules. Leads to mitotic arrest since this prevents microtubules changing from their interphase configuration to a bipolar spindle. This would normally activate the spindle assembly checkpoint. Taxol treatment of normal cells leads to a mitotic arrest that can last hours. After the taxol has been washed out of the body, the normal cell can form its spindle and divide normally. Many cancer cells contain mutations in their checkpoint components. When a cancer cell defective for the spindle assembly checkpoint is treated with taxol, it fails to arrest. Instead, the tumor cell attempts to divide without a bipolar spindle. Seperase is activated and sister chromatids detach. However, without a spindle, chromosome segregation to daughter cells is random. The resulting daughter cells are so defective that they die. Thus the spindle assembly checkpoint protects normal cells treated with taxol but allows tumor cells to undergo a catastrophic attempt at dividing that leads to death of the tumor cells
.
Cyclin activation must occur in order throughout the cell cycle so that specific targets are phosphorylated in specific orders. For each of the following steps in the cell cycle, chose a cyclin and its target.
Which cyclin is used to initiate DNA synthesis, and what is its target?
Choose from the following options.
Cyclin D and its target is MCM helicase
Cyclin E and its target is cdc6
Cyclin B and its target is Rb
Cyclin A and its target is cdc6
Cyclin A and its target is Rb
regulates the initiation of S phase. CyclinE/CDK targets discussed include a DNA helicase, Cdc6, and centrosome component
CyclinE/CDK also phosphorylates Cdc6 to fire the origins of replication.
Which cyclin is used to prevent the second initiation of S-phase? What is its target?
Choose from the following options.
Cyclin D and its target is Rb
Cyclin B and its target is Cyclin E
Cyclin A and its target is cdc6
Cyclin E and its target is cdc6
Cyclin A and its target is Rb
Cyclin A and its target is cdc6
CyclinA that is at high levels after the initiation of S phase until late G2. It is responsible for preventing the cell cycle from going backward or reinitiating S phase. CyclinA/CDK targets discussed include E2F and Cdc6
Which cyclin is used to make sure the cell has grown enough before passing the START point, and what is its target?
Cyclin D and its target is Rb
Cyclin E and its target is wee1
Cyclin B and its target is wee1
Cyclin A and its target is cdc25
Cyclin D and its target is wee1
Cyclin D and its target is Rb
cyclinD: The cyclin responsible for moving the cell past START. CyclinD/CDK targets discussed include Rbb
When E2F is active, cyclinE and cyclinA are made. However, in early G1, a protein called Rb binds to and inactivates E2F, repressing (turning off) the cyclinE and cyclinA genes (Figure 7.13b). In late G1, the cell decides to enter the cell cycle by activating cyclinD/CDK activity. CyclinD/CDK phosphorylates Rb. Phosphorylated Rb can no longer bind or repress E2F. Thus when cyclinD/CDK is active at START, E2F is activated, the cyclinE gene is turned on, and after a short delay, the cyclinA gene is activated
Which cyclin is used to initiate anaphase onset, and what is its target to initiate anaphase onset?
Choose from the following options.
Cyclin B and its target is seperase
Cyclin B and its target is cdc20
Cyclin B and its target is securin
Cyclin D and its target is securin
Cyclin D and its target is seperase
Cyclin B and its target is cdc20
The target of cyclinB/CDK that induces anaphase onset is Cdc20.
1.When Cdc20 is phosphorylated by cyclinB/CDK, it becomes active and begins to ubiquitinate its targets. The major target of Cdc20 to induce sister chromatid separation at anaphase onset is Securin (protein that binds to and inhibits Seperase.
2.CyclinB/CDK phosphorylates Cdc20, which then degrades Securin, releasing Seperase to cleave Scc1 and open the ring connecting sister chromatids. In this manner, all the sister chromatids are separated simultaneously at anaphase onset
The spindle assembly checkpoint ensures that all pairs of sister chromatids are aligned on the metaphase plate. How does it work?
Mad1 and Mad2 activates chk2 kinase, which stabilizes p53
Mad1 and Mad2 induces ubiquitination of cyclinB, inducing anaphase
Mad1 and Mad2 sequesters cdc25, blocking phosphorylation of CDK
Mad1 and Mad2 blocks dynein from moving kinetochores to the metaphase plate
Mad1 and Mad2 senses bipolar tension across sister kinetochores
Mad1 and Mad2 senses bipolar tension across sister kinetochores**
When the rapid DNA damage checkpoint is activated in G2, which of the following occurs?
Choose from the following options.
All DNA is replicated
Cdc25 is active
Cdc25 is in the cytoplasm
Chk1 is in the nonphosphorylated form
Wee1 activity is activated by chk1
Cdc25 is in the cytoplasm
Chk1 kinase functions to delay the cell cycle by phosphorylating Cdc25 (protein phosphatase that removes inhibitory phosphorylations from CDK, activating cyclin/CDK activity)
Once Cdc25 is phosphorylated, it binds to an adaptor protein called 14-3-3 and is exported out of the nucleus and into the cytoplasm where it is inactive
cdc20 plays a central role in progression through the cell cycle. Different proteins have different effects on cdc20. Which of the following statements best describes cdc20?
Choose from the following options.
cdc20 is a kinase that is inhibited by the chk1 kinase and stimulated by CDK.
cdc20 is an ubiquitin ligase that is activated by CDK and inhibited by Mad2.
cdc20 is an ubiquitin ligase that is phosphorylated by cdc25 and inhibited by DNA damage.
cdc20 is a phosphatase that removes the phosphate on Thr 161 of CDK that was placed there by wee1 kinase
cdc20 is a phosphatase that is inhibited by DNA damage and activated by CDK
cdc20 is an ubiquitin ligase that is activated by CDK and inhibited by Mad2.
A variety of important model organisms have elucidated different aspects of the cell cycle. The molecular nature of cyclins and the fact that their levels oscillate in the cell cycle was discovered using ______________.
Choose from the following options.
Genetics in the fission yeast S. pombe
Biochemistry in Sea Urchin embryos
Biochemistry in Xenopus oocytes
Biochemistry in mammalian tissue culture cells
Genetics in C. elegans
Biochemistry in Sea Urchin embryos
Which of the following mutations would cause a metaphase arrest?
Choose from the following options.
A null mutation in Securin
A Ser to Ala mutation in Securin
A Lys to Ala mutation in Securin
A Ser to Ala mutation in Seperase
A Lys to Ala mutation in cdc20
A Lys to Ala mutation in Securin
Lysine and Alanine have different properties as amino acids, which may lead to very different protein function compared to Ser and Ala, which have similar non polar proerpties and may not lead to a drastic mutaiton. Securin is a protein that binds and inhibits Seperase, which targets Scc1 to open cohesin at anaphase onse
MDM2 normally directly inhibits p53 activity by destabilizing p53. You would therefore predict that MDM2 has which of the following properties?
Choose from the following options.
MDM2 is a proto-oncogene
MDM2 is a tumor suppressor gene
MDM2 activity increase apoptosis
MDM2 activity slows down the cell cycle
MDM2 activity increases p21 and p27
When active, p53 functions as a tumor suppressor gene. So, if MDM2 inhibits a tumor suppressor gene, it acts as a proto-oncogene