alkanes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
what are alkanes?
homologous series of saturated hydrocarbons w/ the general formula CnH2n+2
how reactive are alkanes?
very unreactive, but do burn and react w/ halogens (to form haloalkanes)
what is a homologous series?
family of compounds:
same general formula
similar chemical properties
each member differs by the addition of a CH2 group and a gradual change in physical properties
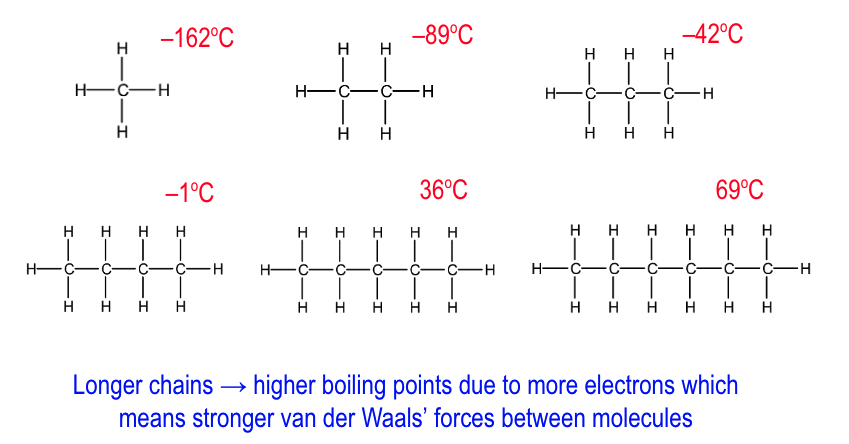
how does bpt change as the C chain gets longer?
longer C chain = higher bpt
as more e-
so stronger VDWs between molecules
which require more energy to break
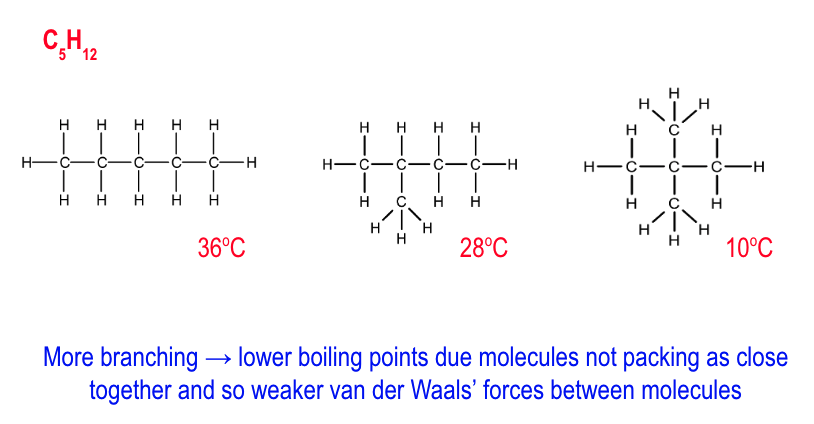
how does bpt change w/ increase in branching?
more branching = lower bpt
as molecules do not pack as close together
so weaker VDWs between molecules
which require less energy to break
how does viscosity change as the C chain gets longer?
viscosity increases (can be found by residue in fractional distillation)
how does flammability change as the C chain gets longer?
flammability decreases
how does the flame change as the C chain gets longer?
flame gets dirtier (clean → smoky flame)