ACS (FINAL OCHEM)
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
Chapter 1: Structure: shape and stability
Molecular Orbital diagrams
Aufbau principle = fill the lowest energy first
Pauli Exclusion principle = only two electrons per orbital
hund’s rule = Orbitals of the same energy receive one electron
Fill the lowest energy orbital
No orbital may hold more than two electrons, and they must be of opposite spin
When filling degenerate orbitals (orbitals of the same energy), each orbital receives one electron, then the degenerate orbitals receive a second electron of the opposite spin
AFTER hybridization - only one row (put one spin on each line firs,t then fill the others
BEFORE hybridization - 2 or more rows (fill the lowest line before moving to higher lines
Look up atom in periodic table - column # = valence electrons
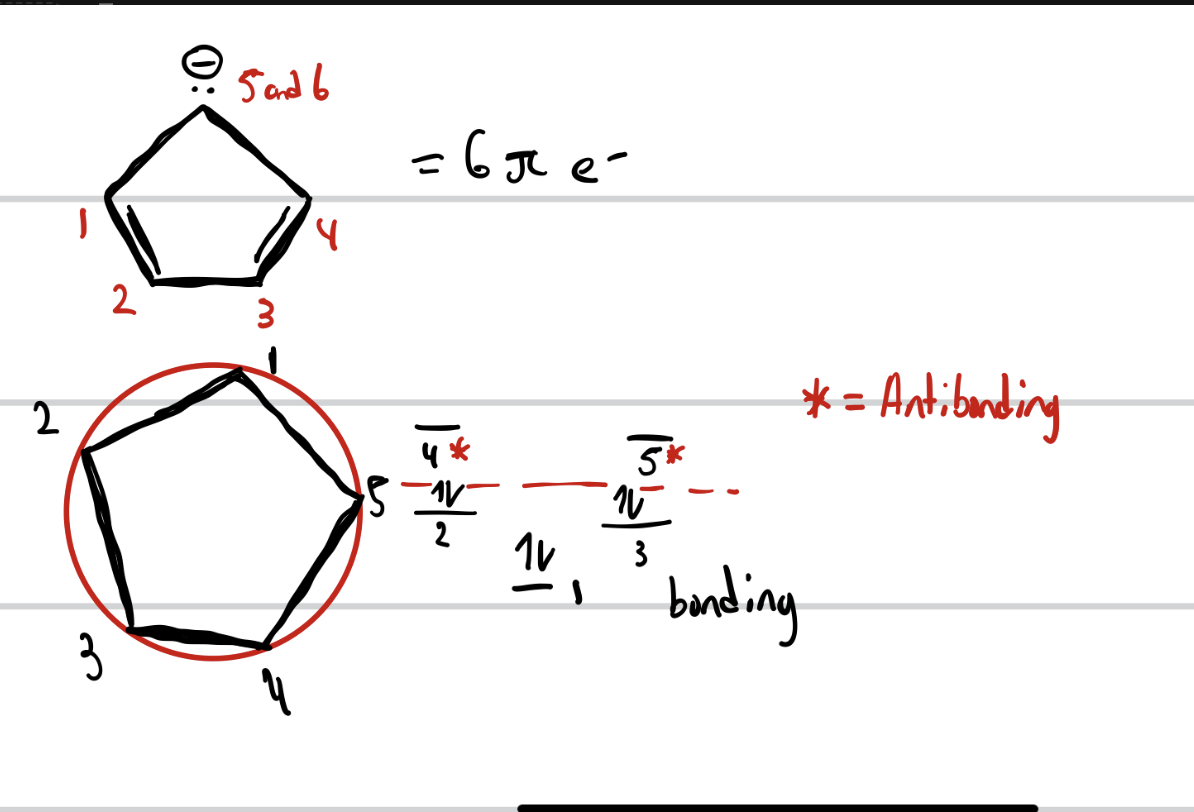
VSEPR Theory
Biggest resonance Contributor
1. All atoms obey the octet rule (especially for second-period elements like C, N, O, F).
Atoms should not exceed or fall short of 8 electrons (unless it's an exception like P or S).
2. Minimal formal charges.
The most stable structure has the fewest formal charges.
A neutral molecule is generally more stable than charged forms.
3. If charges exist, they’re on appropriate atoms:
Negative charges should be on more electronegative atoms (like O or N).
Positive charges should be on less electronegative atoms (like C).
4. More covalent bonds (double bonds) = more stability, if the octet rule is still satisfied.
A structure with more double or delocalized bonds tends to be more stable if it doesn’t cause an octet violation.
5. Charge separation is minimized.
Structures with separated positive and negative charges are generally less stable than ones where charges are closer together or nonexistent.
6. Avoid like charges on adjacent atoms.
Two positive or two negative charges next to each other is unstable.
7. Delocalization of electrons (especially charges and π electrons).
If a structure spreads out electron density over multiple atoms (especially in conjugated systems), it gains stability.
Carbocation + radical Stability
Benzylic > Allylic > 3 prime > 2 prime > 1 prime > anything on a double bond
Resonance
Remember, it is making and breaking bonds
Negative charge = extra electron pair on the atom
Positive charge = electron pair missing on the atom
Double and triple bonds can move
The most stable resonance structure = Resonance hybrid
All atoms have a full octet, and the most electronegative atom should contain the negative charge
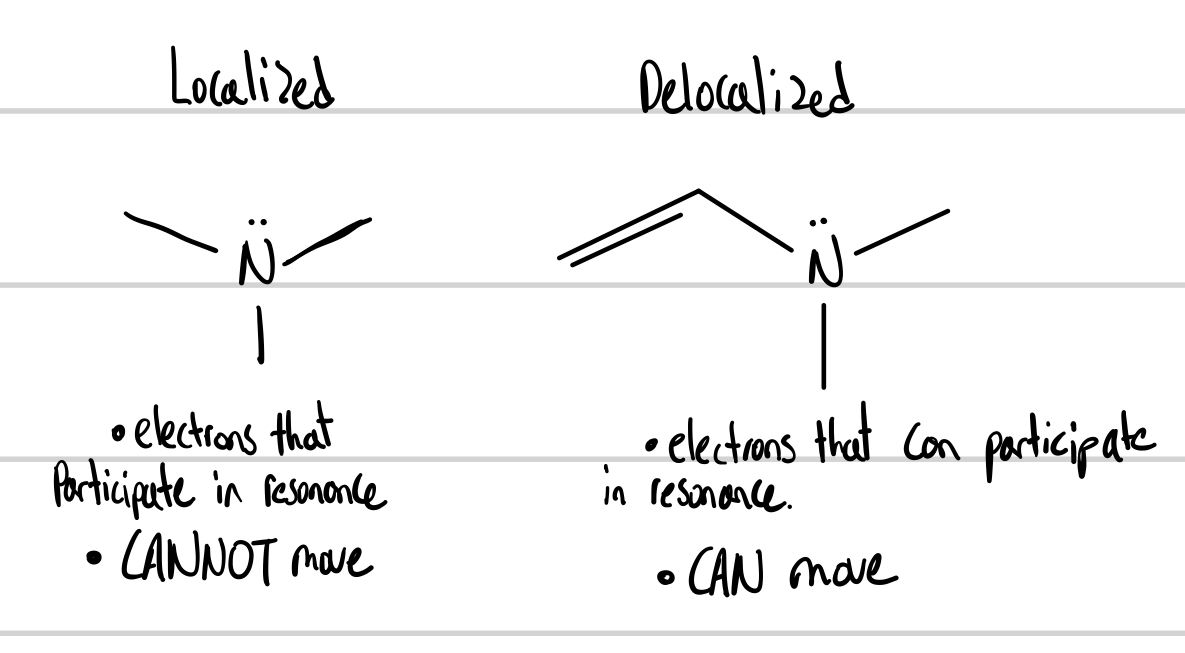
Delocalized VS Localized
Localized
electrons that participate in resonance
Electrons that CANNOT move
Delocalized
electrons that can participate in resonance
electrons that CAN move
If a heteroatom (N,O) has multiple lone pairs, only ONE of those lone pairs is localize the rest are localized and must stay put.
If a heteroatom (N,O) already has a double bond, then any lone pairs still present are localized.
Intensity of dipole moment
the difference of electronegativity
the more balanced the less strength of the dipole
Chapter 2: Structure: Nomenclature and Functional Groups
Functional Groups
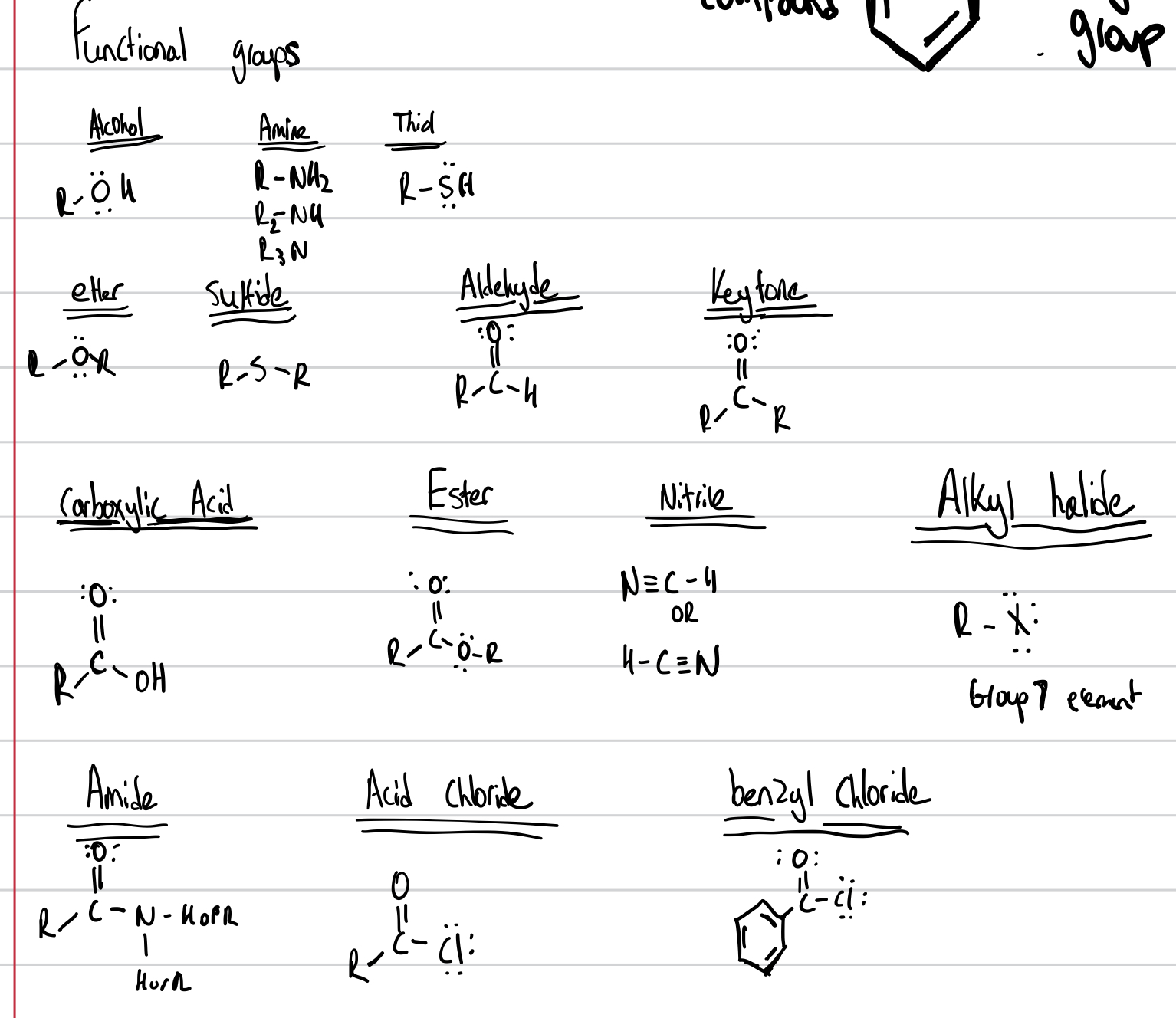
IUPAC Groups
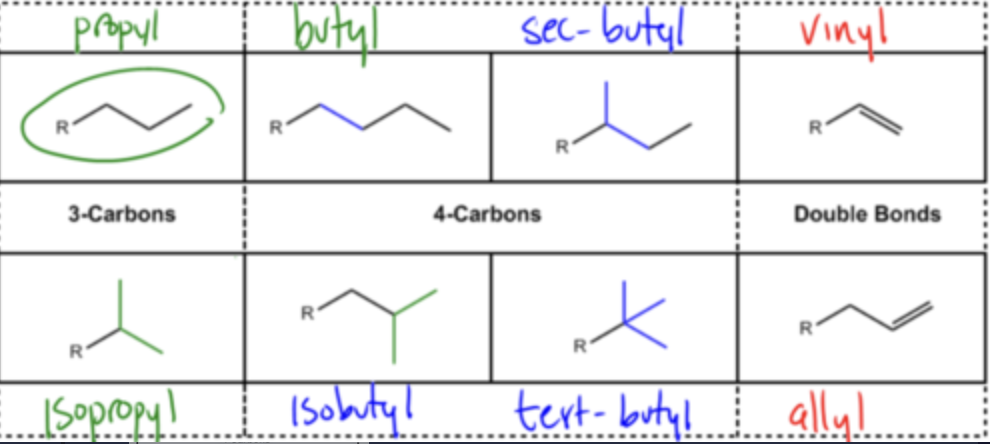
General IUPAC rules
want the alcohol and alkene on the lowest number possible
if it has both alcohol and alkene then the alcohol will be on the lowest carbon
If debating between two ways to start a number and they have a substituent on the same carbon, pick the one that has more substituents.
cycloalkanes = pick the lowest numbering system possible
Prefixes that DON'T Count: di,tri,tetra, sec, tert, iso
Boiling point and IMF
The highest boiling point will be the strongest IMF
Ionic bonding (strongest)
H-bonding
Dipole-dipole
London Dispersion Forces (weakest)
The more spread out the molecule, and the more carbons, the higher the boiling point
know the primary, secondary, and tertiary carbons of the functional groups
for alcohols you count starting from the first carbon and see how many carbon that is connected too
for other functional croups you look directly at what the functional group is connected too
Solubility
Like dissolves in like
Alkanes usually do not dissolve in water : the smallest chain the more likely soluble in water
More carbons mean LESS soluble
<5 carbons = likely SOLUBLE in water
>5 carbons = likely INSOLUBLE in water
Chapter 3: Structure: Isomers
R and S
When assigning priority for R and S, it goes by ATOMIC NUMBER
If the highest priority is dashed (in the plane) then you have to flip it
Chair confirmation
sold wedge = up (axial or equatorial up)
dashed wedge = down ( axial or equatorial down)
the most stable chair is when the groups are equatorial
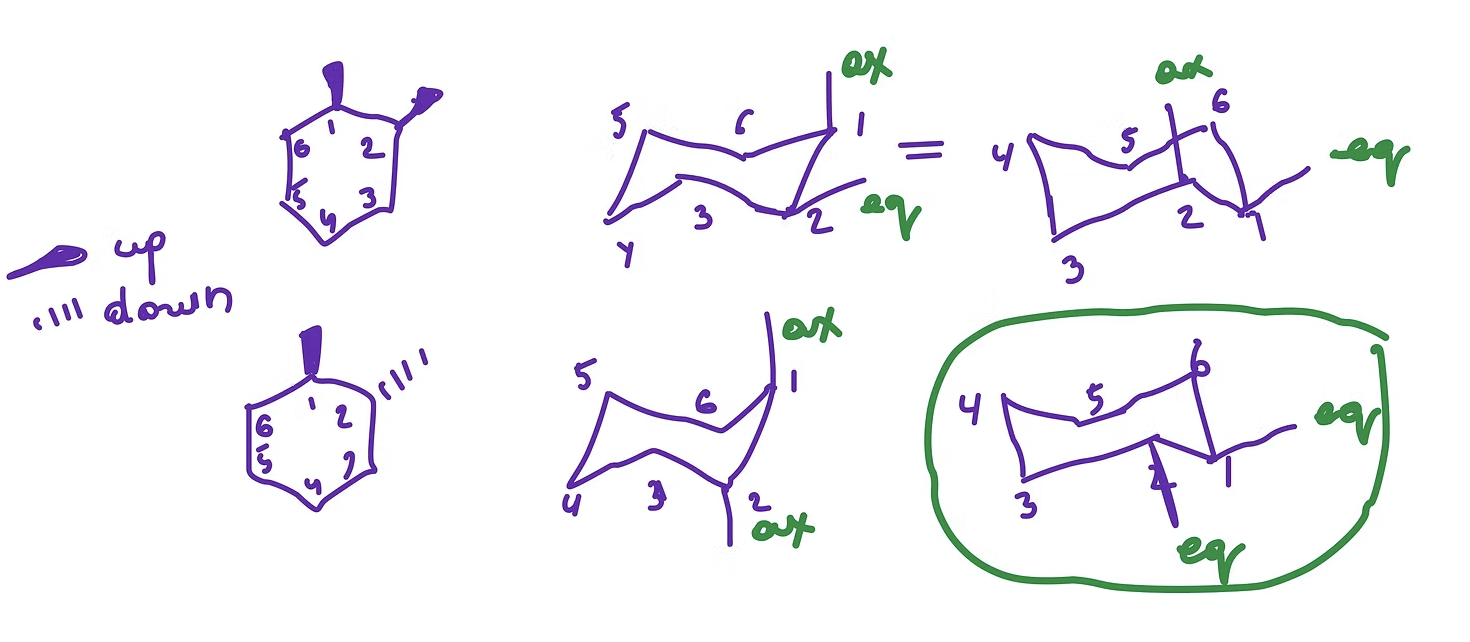
what is the purpose of confirmations
A molecule adopts conformations to reduce its overall energy
observed conformation should focus on minimizing strain, minimizing overall energy
CAUSED by TORSIONAL STRAIN
Ring strain arises from bond angles that are not ideal (sp3 or 109.5°)
When is a compound optically active? (achiral vs chiral)
Chiral = optically active = stereogenic center (non-superimposable)
Achiral = not optically active = no stereogenic center
If the molecule is symmetric then it is optically inactive
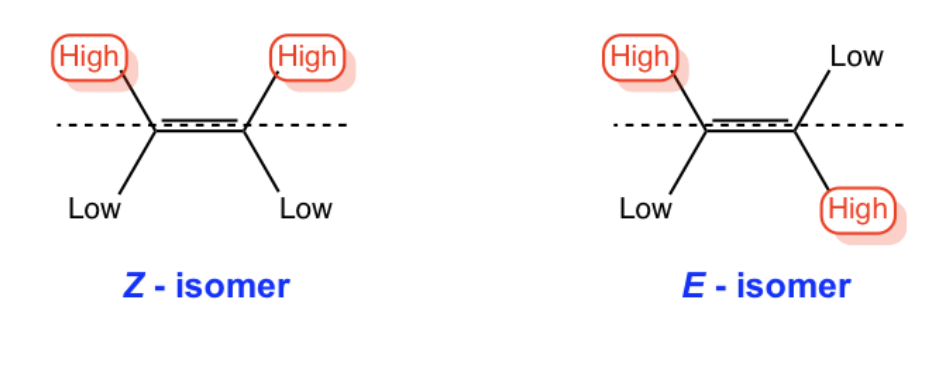
E vs Z
When an alkene exists in a structure, E and Z are used to identify the name.
E = Trans - priority group is on opposite sides
Z = Cis - priority groups are on the same side
ATOMIC NUMBER
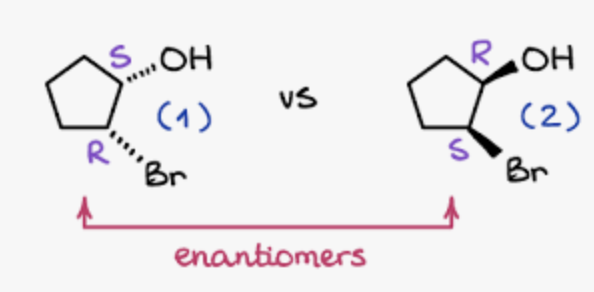
Enantiomers
All stereogenic centers change confirmation
before R,R,S after S,S,R
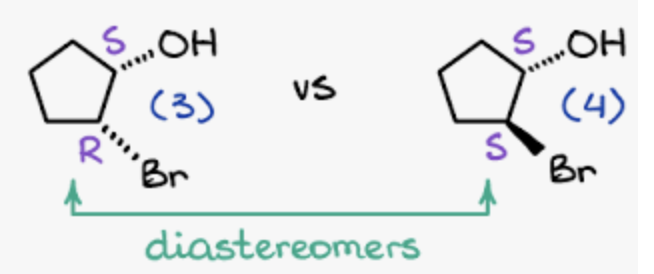
Diastereomers
Some but not all stereogenic centers change confirmation
before R,S vs after S,S
Constitutional Isomers
have the same molecular formulas but have different connectivity
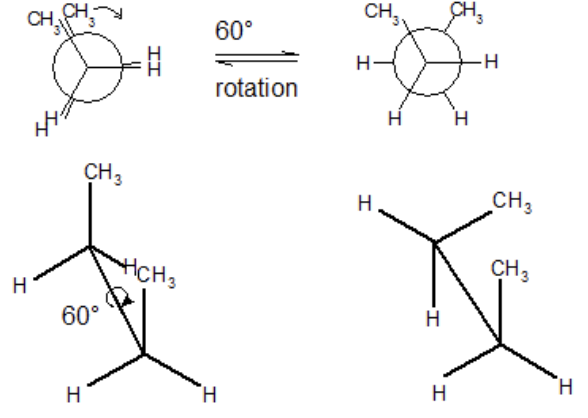
conformational isomers
have the same molecular formula, are connected in the same absolute way, but differ in rotation of single bonds.
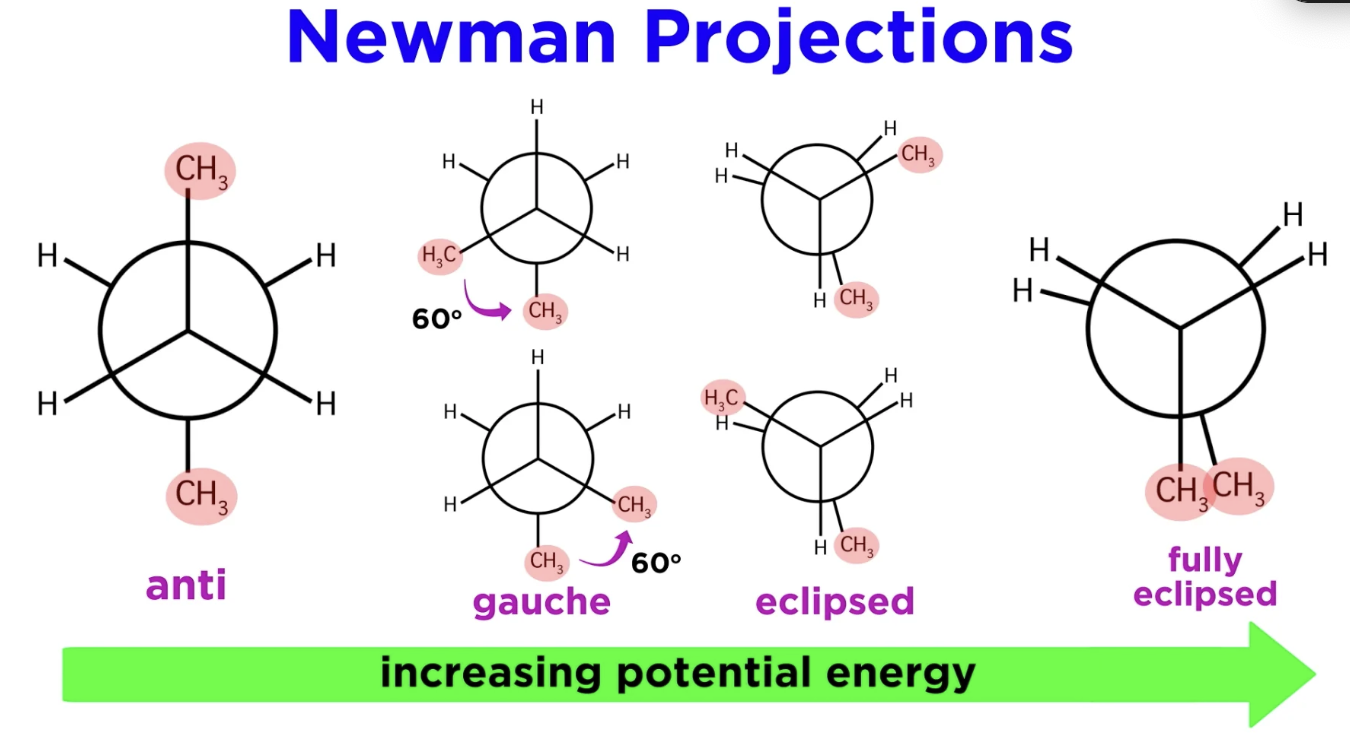
Newman projections
The further apart the bigger groups are and the further apart they are, the more stable.
Less stable = higher energy
Staggered
The substituents on the front and back carbons are as far apart as possible
Eclipsed conformation
The substituents on the front and back carbons are directly aligned. LEAST STABLE
Gauche conformation
staggered conformation where two bulky groups are adjacent to each other (60° apart)
Anti Conformation
bulky groups are opposite from each other
Ring Strain
The instability in cyclic compounds due to angular distortion and torsional strain
results in an increased energy and reactivity
Ring Strain = bond angles INTERNAL to the ring
Torsional strain
when atoms or groups of atoms are eclipsed causing increased energy (less stable)
Steric Strain
strain caused by two bulky groups that are close together
electron clouds experience a repulsion causing increased energy and destabilization in the molecule.
Fisher Projection
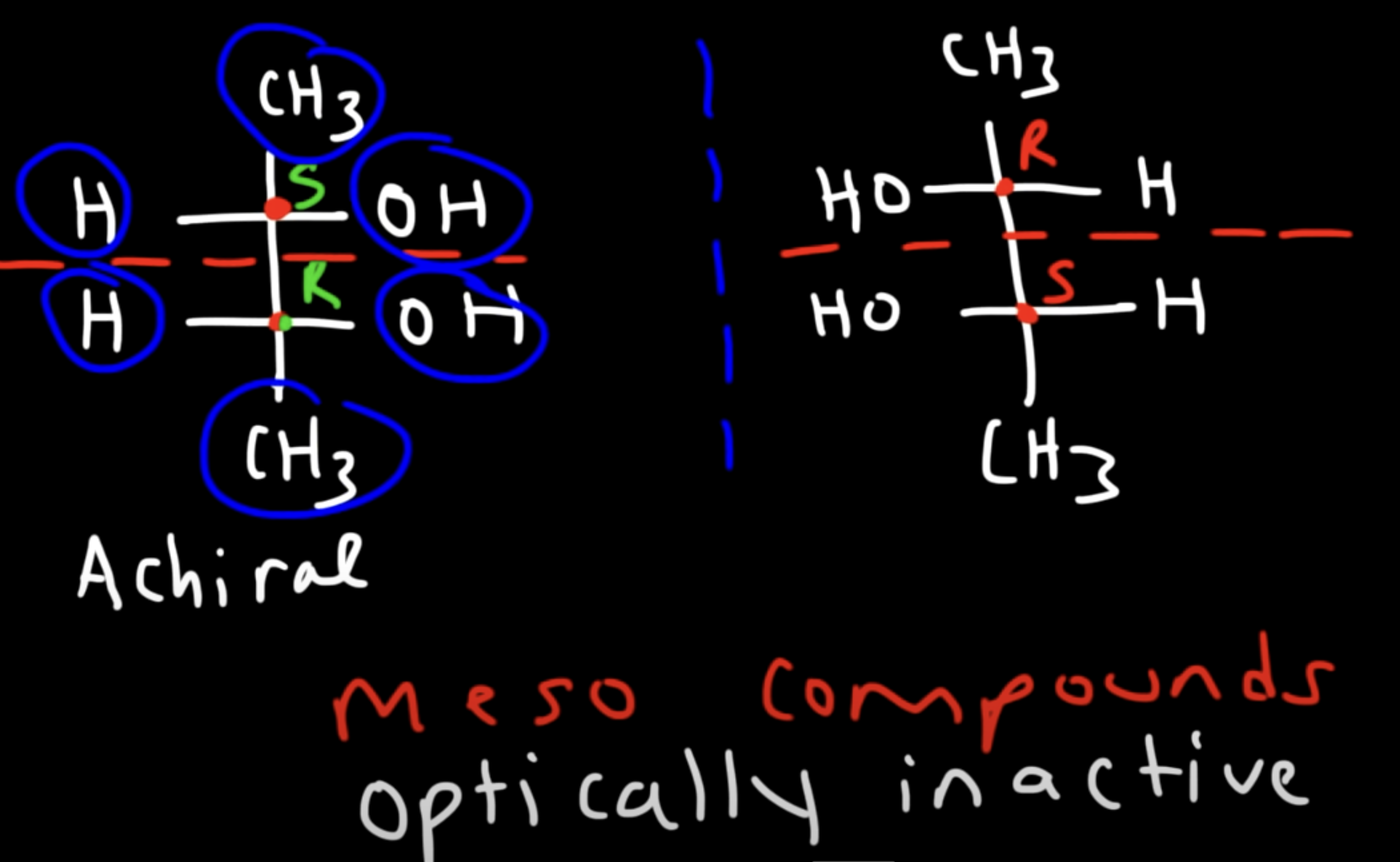
Meso compounds
have chiral centers but have a plane of symmetry, making them achiral
optically inactive
R and S or S and R
Chapter 4: Acids and Bases
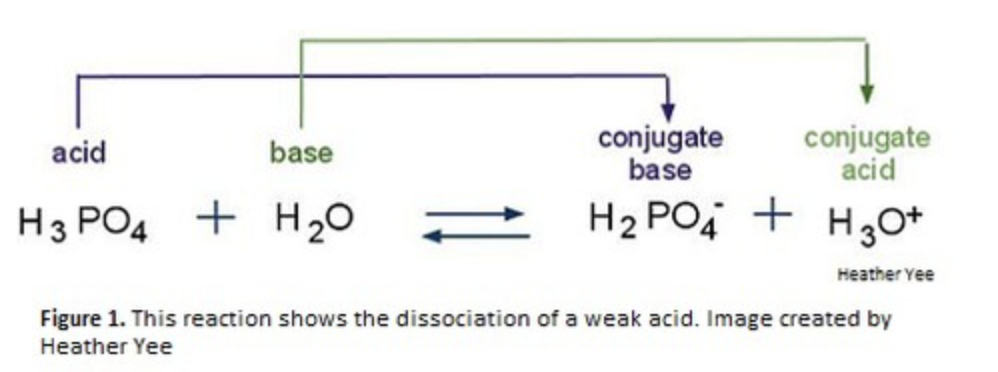
Bronsted lowry acids and Bronsted Lowry Bases
Bronsted Lowry Acid = proton donor
Bronsted Lowry Base = proton acceptor
only one starting material contains a hydrogen it must be the acid. If only one starting material has a lone pair or a pi bond it must be the base
starting material with a positive charge = acid
starting material with a negative charge = base
Lewis Base VS Lewis Acids
Lewis base = donate a pair of electrons
Lewis acid = accepts a pair of electrons
Radicals cannot accept an electron pair
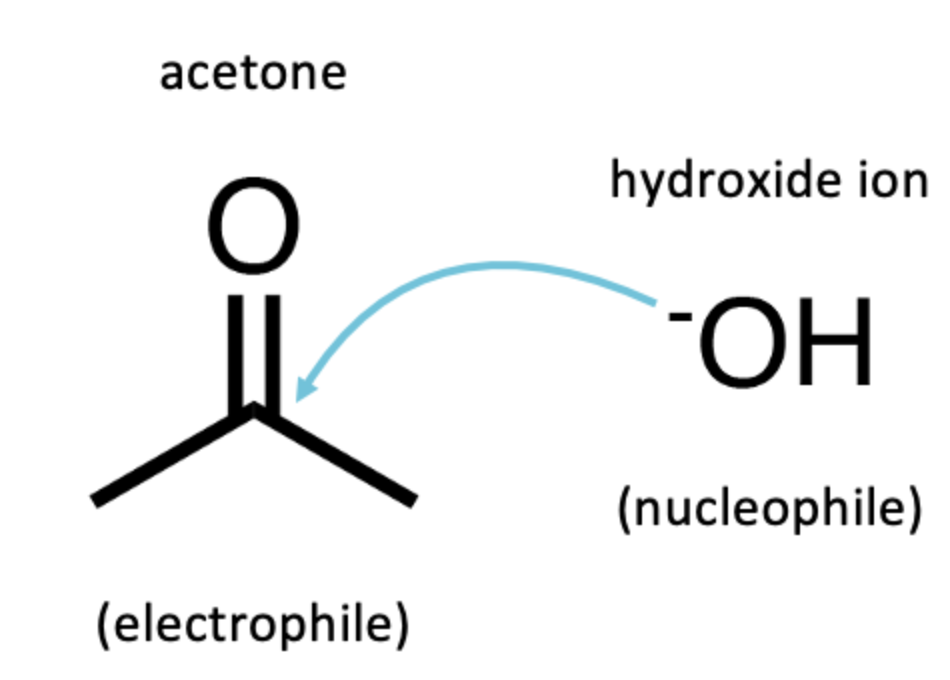
Nucleophile VS Electrophile
Nucleophile - electron-rich
generally have a negative charge
Electrophile - electron-poor
generally have a positive charge
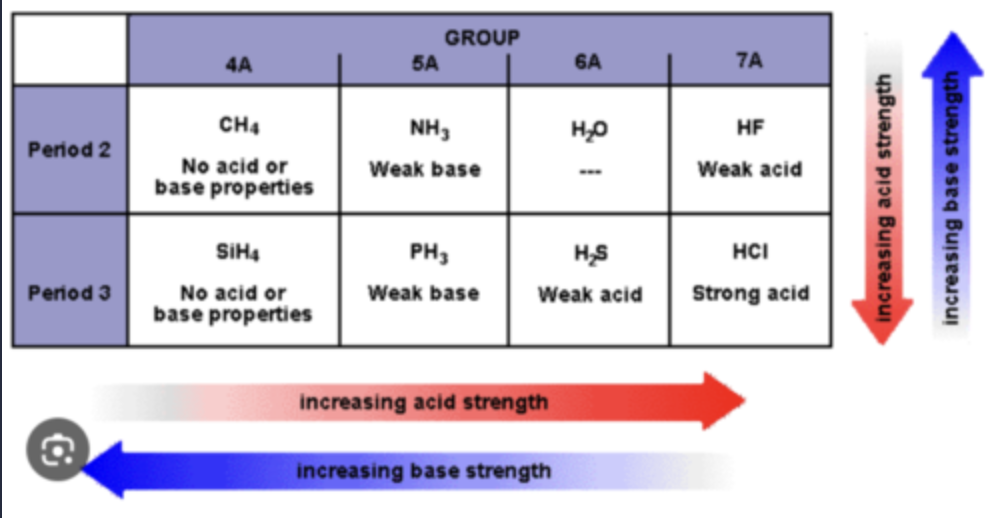
Factors that Determine Acid Strength
Element Effects - more electronegative = stronger acid
Inductive effects - the closer the halide is to an anion and the more electronegative = the stronger the acid
Resonance effects - if more resonance is available = then the stronger the acid
Hybridization effects - acid strength sp3, sp2, sp
Ph and pka + equilibrium
Higher value (Ph and pka) = Basic
K - equilibrium constant [products/reactants]
If the products are more basic than K>1
If the reactants are more basic than K<1
Acid/ Base Reactions
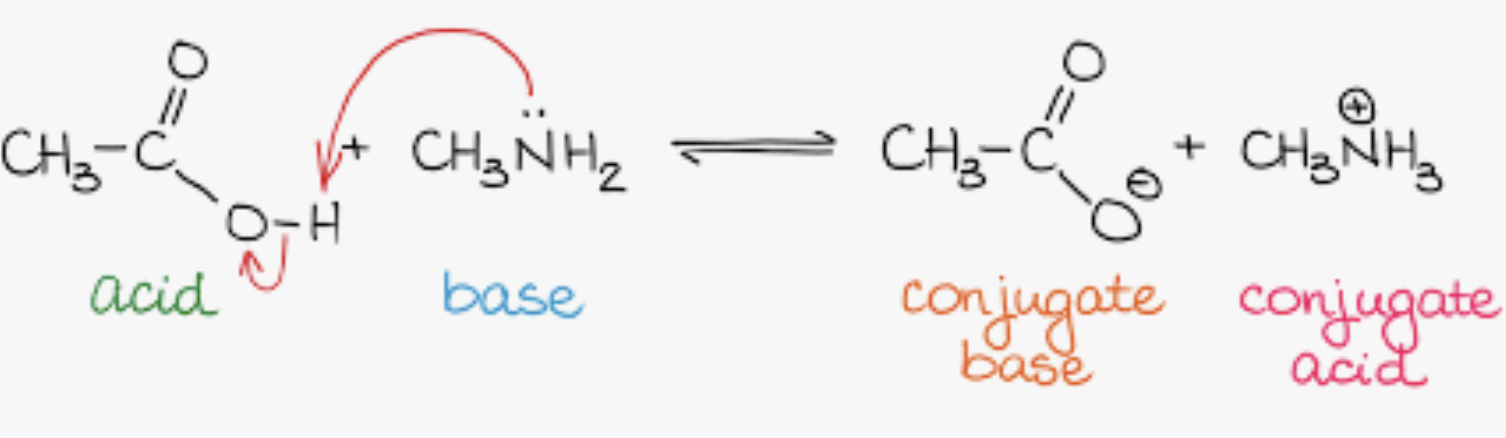
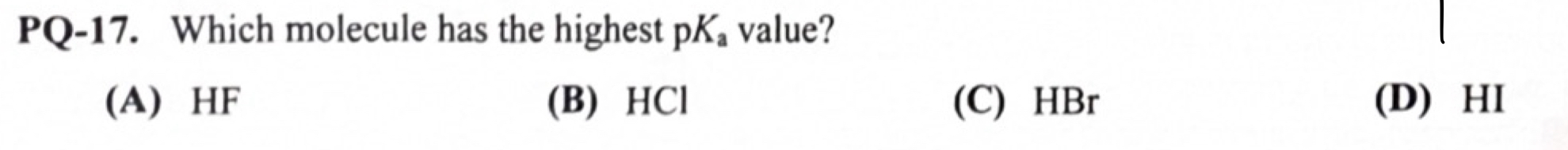
highest pka will be the most basic - HF
Chapter 5: Nucleophilic Substitution Reactions
Acid/Base Reactions
SN2
Bimolecular
One step = second order
Changing the concentration of either reactant impacts the rate
Aprotic
Rate law = K [Alkyl Halide][Nucleophile]
Do R and S (ONE PRODUCT)
1° and 2° (good NU) alkyl halides undergo SN2 reactions with ease
opposite specific rotation
Inversion of configuration
Most reactive → methyl, 1°,2°, and 3°
PBr3 and SOCl2 ALWAYS SN2 (inversion of stereochemistry)
HBr 1° = SN2
![<ul><li><p>Bimolecular</p></li><li><p>One step = second order</p></li><li><p>Changing the concentration of either reactant impacts the rate</p></li><li><p>Aprotic</p></li><li><p>Rate law = K [Alkyl Halide][Nucleophile]</p></li><li><p>Do R and S (ONE PRODUCT)</p></li><li><p>1° and 2° (good NU) alkyl halides undergo SN2 reactions with ease</p></li><li><p>opposite specific rotation</p></li><li><p>Inversion of configuration</p></li><li><p>Most reactive → methyl, 1°,2°, and 3°</p></li><li><p>PBr3 and SOCl2 ALWAYS SN2 (inversion of stereochemistry)</p></li><li><p>HBr 1° = SN2</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/78f1b387-8bc1-41eb-a119-0b36496c3909.png)
SN1
Unimolecular
two step = first order
polar protic
Rate law = K [Alkyl Halide]
forms a racemic mixture
2° and 3° alkyl halides undergo SN1 reactions
Specific rotation is zero
Most reactive → 3°, 2°, 1°
HBr 2° and 3° → SN1
![<ul><li><p>Unimolecular</p></li><li><p>two step = first order</p></li><li><p>polar protic</p></li><li><p>Rate law = K [Alkyl Halide]</p></li><li><p>forms a racemic mixture</p></li><li><p>2° and 3° alkyl halides undergo SN1 reactions</p></li><li><p>Specific rotation is zero</p></li><li><p>Most reactive → 3°, 2°, 1°</p></li><li><p>HBr 2° and 3° → SN1</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b4e30396-d350-4e4d-9837-a4e6c8567caa.png)
Nucleophile strength
Lewis Bases are nucleophiles = electron-rich
Negative charged nucleophiles react the fastest
nucleophilic strength in the same period (row), the further left, the stronger the nucleophile
nucleophilic strength in the same family (column), the further down the stronger the nucleophile
What Makes a Good Leaving Group
The weakest Bronsted-Lowry base/ strongest conjugate acid is what makes the best leaving group
Large polarizable anions make good leaving groups
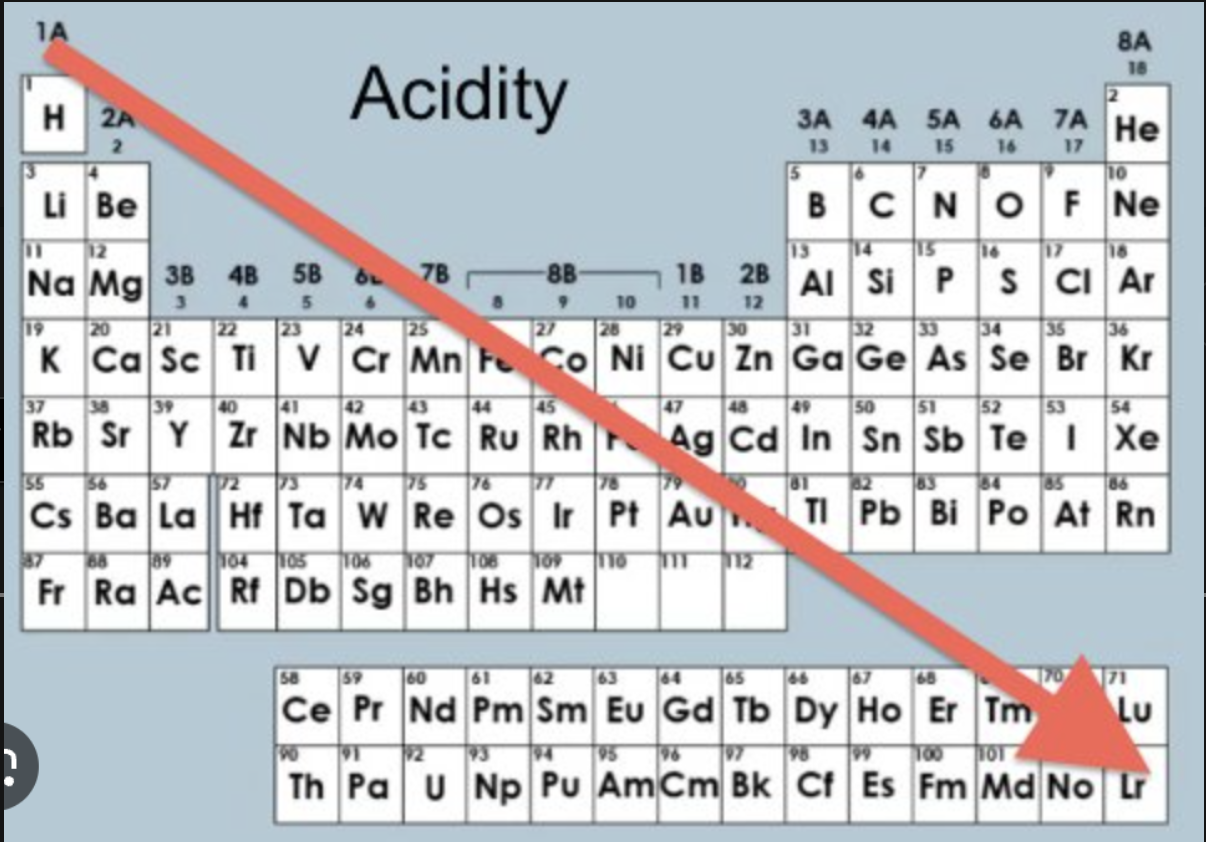
Solvolysis
-Tertiary (3°) > Secondary (2°) > Primary (1°)
-Allylic or Benzylic (resonance-stabilized) structures also favor solvolysis.
SN1: The leaving group departs first, creating a carbocation intermediate. The rate of reaction depends on the stability of the carbocation.
SN2: The nucleophile (solvent) attacks the substrate in one step, so the substrate must be less hindered.
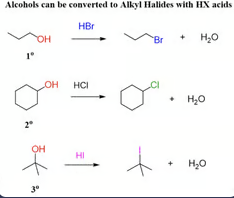
Mechanism of HX reaction with alkyl alcohols
rearrangements can occur (hydride or alkyl shift) when a more stable carbocation can be formed from the initial carbocation
Only carbocation rearrangement in SN1 (2° and 3°)
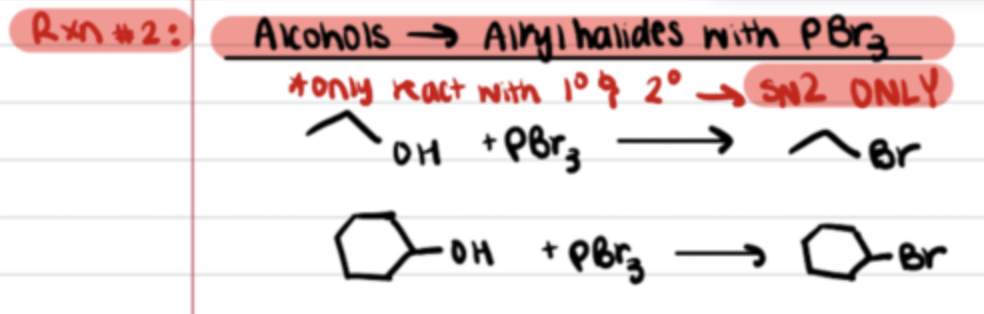
Reaction of Alcohols to alkyl halides with PBr3
Inversion of Stereochem (SN2)
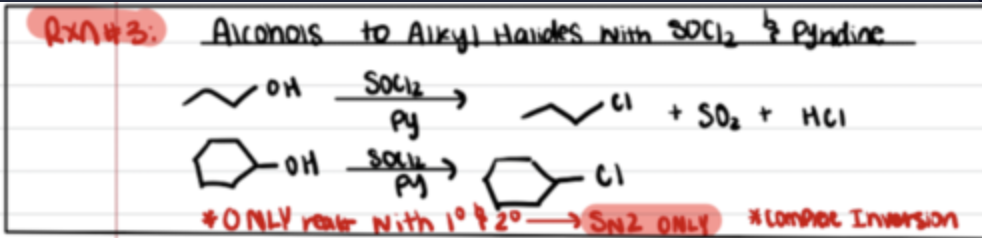
Reaction of alcohols to alkyl halides with SOCl2 and Pyriedine
Inversion of Stereochem (SN2)
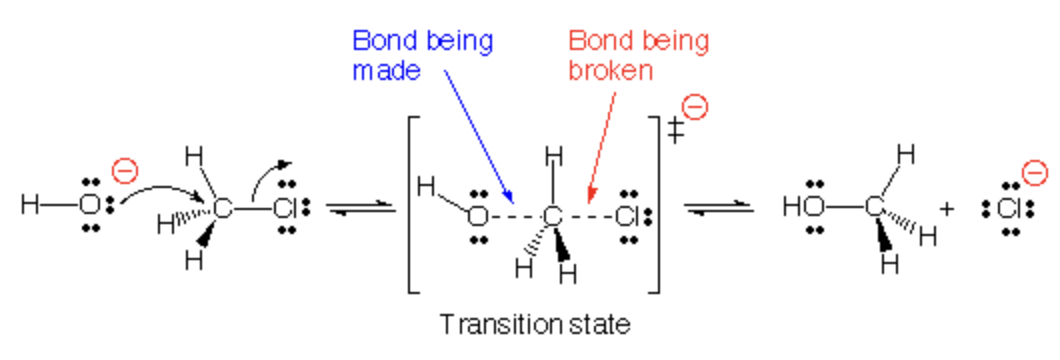
Transition state for SN2
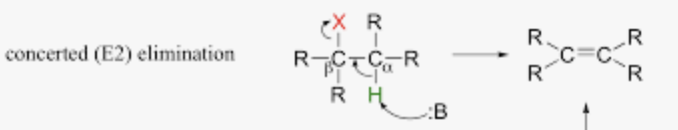
One bond is being broken, and one bond is being made, ONE STEP
Halide is being broken and addition of nucleophile
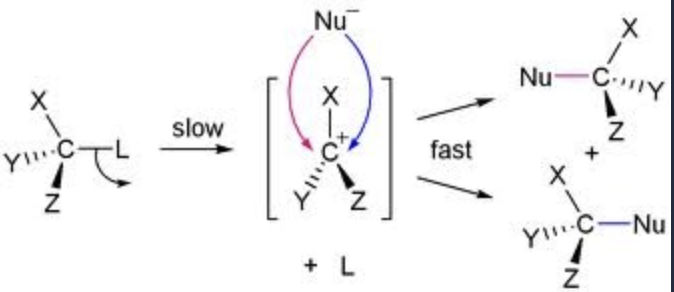
Transition state for SN1
Two Step reaction
first step is the bond being broken and the second step is the addition of the nucleophile
Polar Protic VS. Polar Aprotic
Polar protic: H-bonding- H2O, ethanol, and methanol- a strong base is a weak NuPolar
Aprotic: NO H-bonding- DMSO, DMF, and acetone- a strong base is a strong Nu
Chapter 6: Elimination Reactions
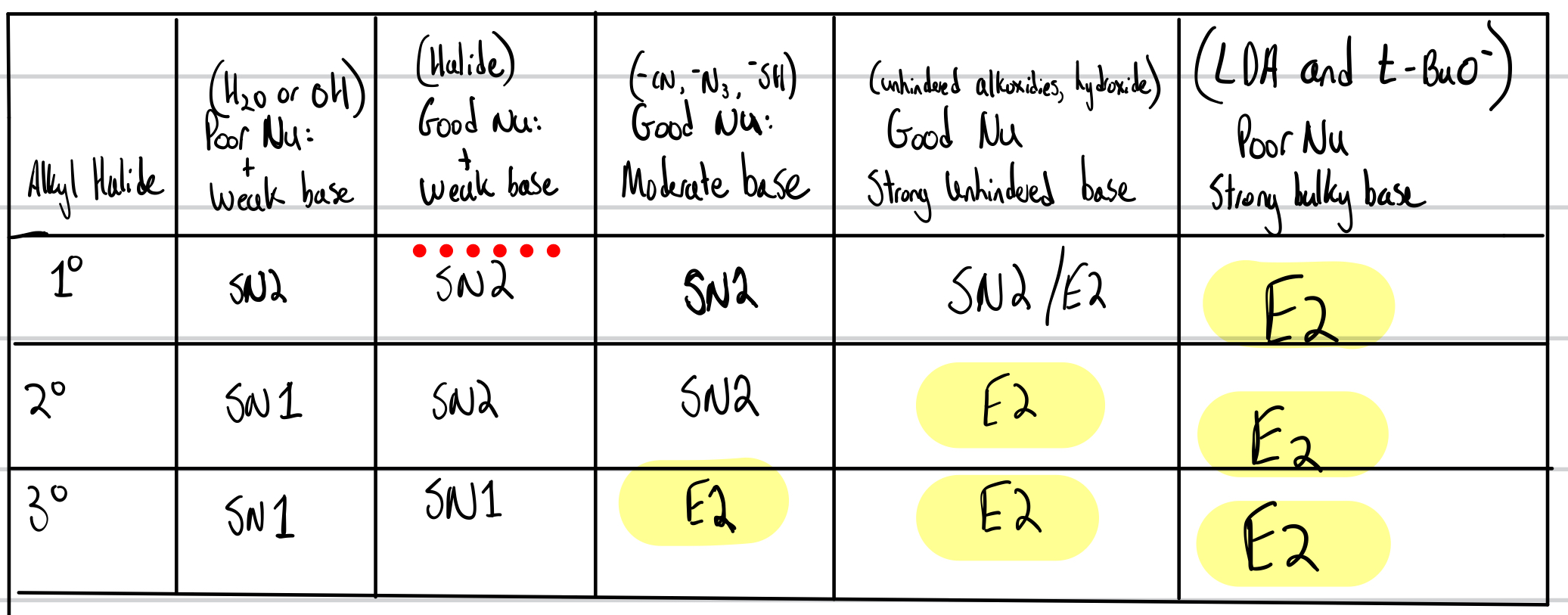
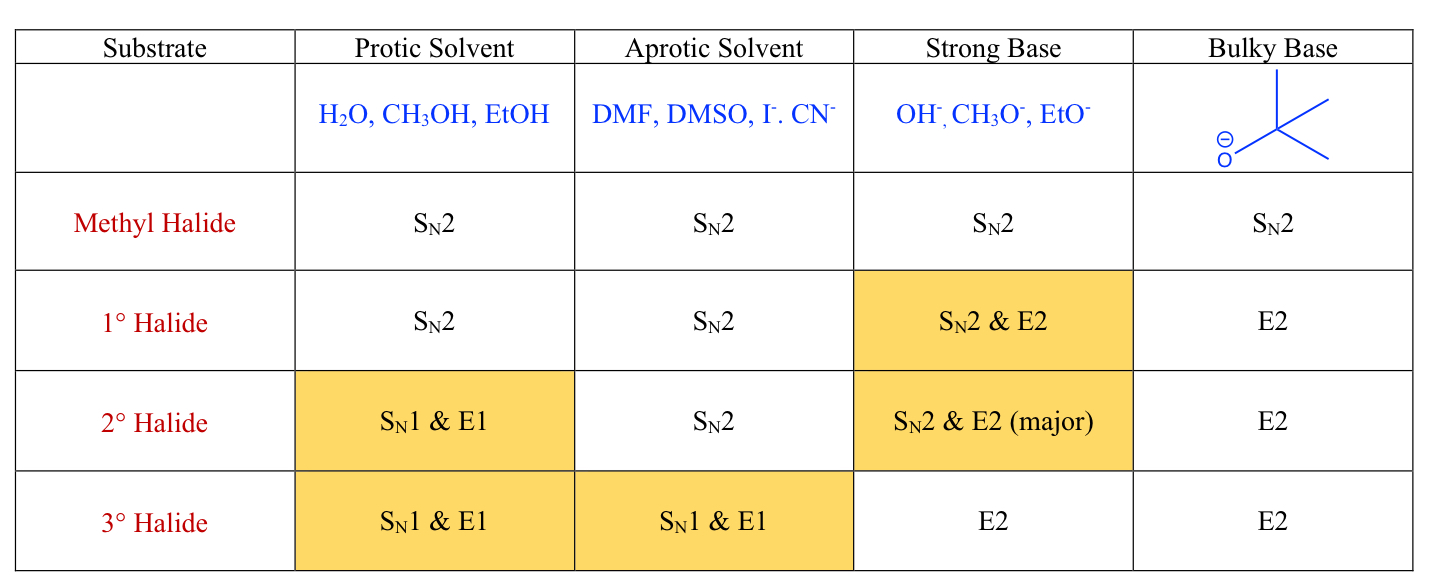
E1 And E2 Chart
E2:
One-step mechanism
2nd order = bimolecular
Better leaving group = faster reaction (strong conjugate acid/ weaker base)
Polar aprotic (no H-bonding) increases the rate of the reaction
E or Z
If a bulky base, then the less substituted product is preferred
Rate = [Alkyl Halide][Nucleophile]
E1:
Two-step reaction mechanism
First order = Unimolecular
better leaving groups
Carbocation Rearrangements can occur for E1
Polar protic (H-bonding) increases the rate of reaction
Rate= [Alkyl Halide]
![<p>E2:</p><ul><li><p>One-step mechanism</p></li><li><p>2nd order = bimolecular</p></li><li><p>Better leaving group = faster reaction (strong conjugate acid/ weaker base)</p></li><li><p>Polar aprotic (no H-bonding) increases the rate of the reaction</p></li><li><p>E or Z</p></li><li><p>If a bulky base, then the less substituted product is preferred</p></li><li><p>Rate = [Alkyl Halide][Nucleophile]</p></li></ul><p></p><p>E1:</p><ul><li><p>Two-step reaction mechanism</p></li><li><p>First order = Unimolecular</p></li><li><p>better leaving groups</p></li><li><p><strong>Carbocation Rearrangements</strong> can occur for E1</p></li><li><p>Polar protic (H-bonding) increases the rate of reaction</p></li><li><p>Rate= [Alkyl Halide]</p></li></ul><p></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/674839db-e604-4ae2-b38e-851ded35e14e.jpg)
Zaitsev’s Rule
the most substituted for E1 and E2 are generally favored
Want conjugation
EXCEPT when a bulky base is used (CH3)3 OH tert-butoxide
If CIS = two products form
if TRANS = less substituted product is preferred
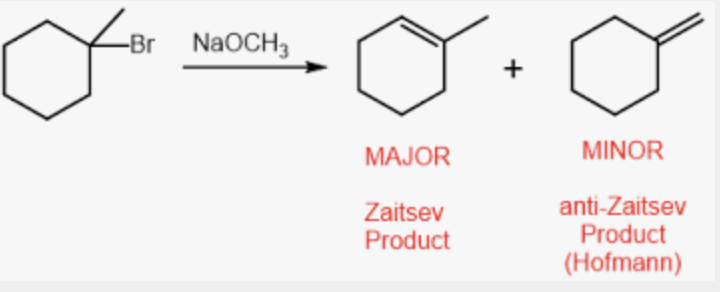
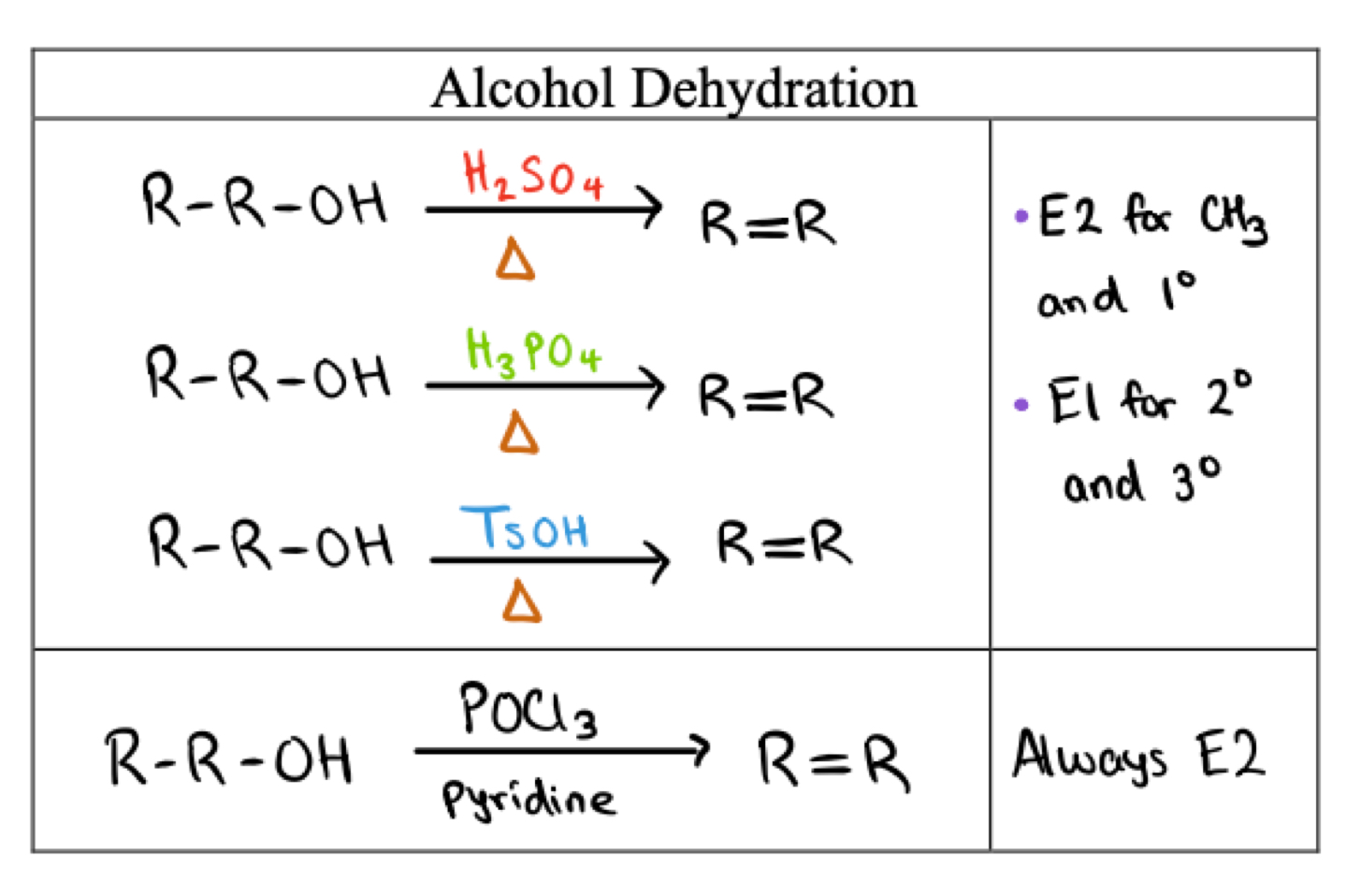
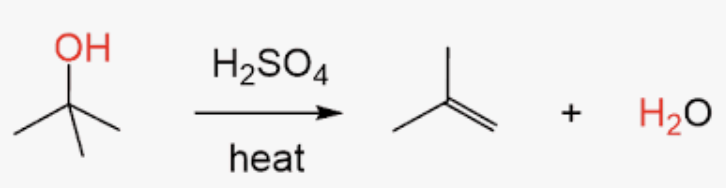
Dehydration of alcohols
3° and 2° follow E1 mechanism (Carbocation Rearrangment)
first step is protonation of the alcohol
E2 Mechanism
E1 Mechanism
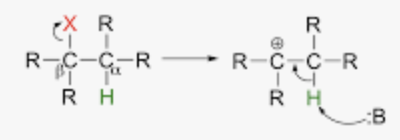
Chapter 7: Addition Reactions to Alkenes and Alkynes
Addition of Alkenes - R=R with HX (Hydrohalogenation)
follows Markonikov’s rule
Two Steps:
1) Nucleophilic attack
can do shifts, carbocation rearrangements
X = Cl, Br, I
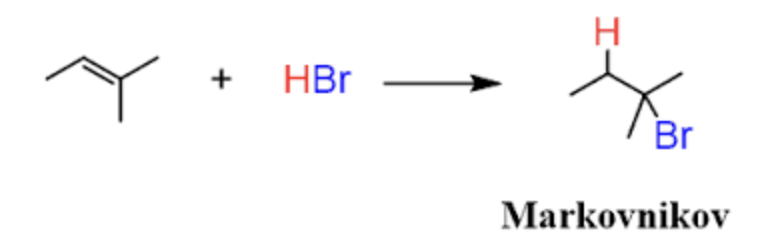
Markovnikov’s Rule
Hydrogen will add to the least substituted carbon of the double bond
Addition of Alkenes - R=R with H2O and H2SO4 or HEt, H+ (Hydration)
Formation of an Alcohol on the most substituted carbon
Adding water/acid catalyst
H2O/H+ or H2O/H2SO4+
Markovnikov’s Rule
CAN DO SHIFTS/ REARRANGEMENTS
three steps
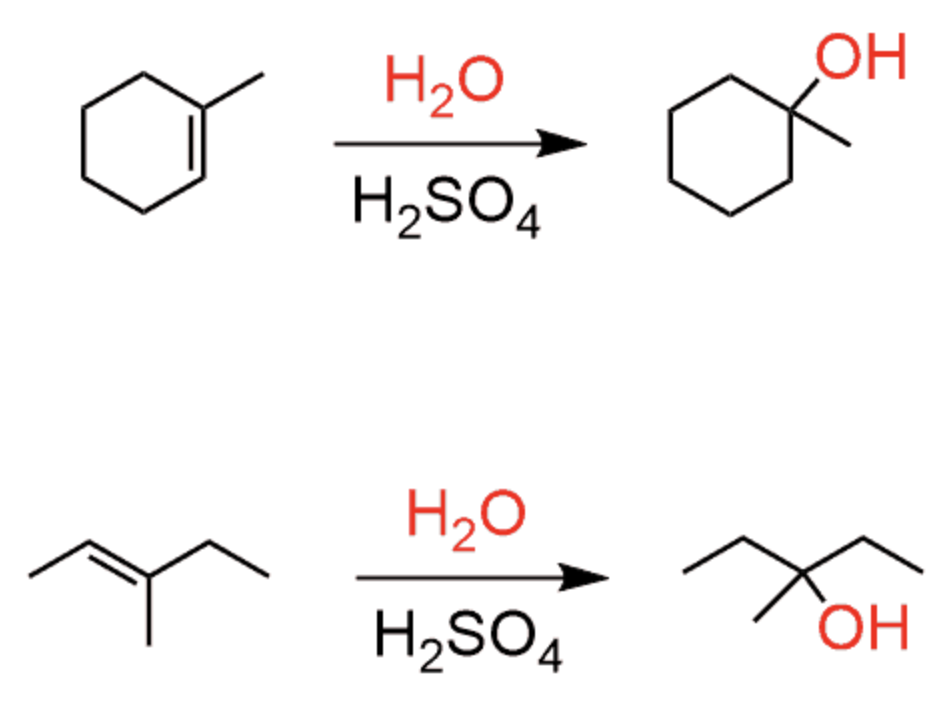
Addition of Alkenes - R=R with Br2 or Cl2 (Halogenation)
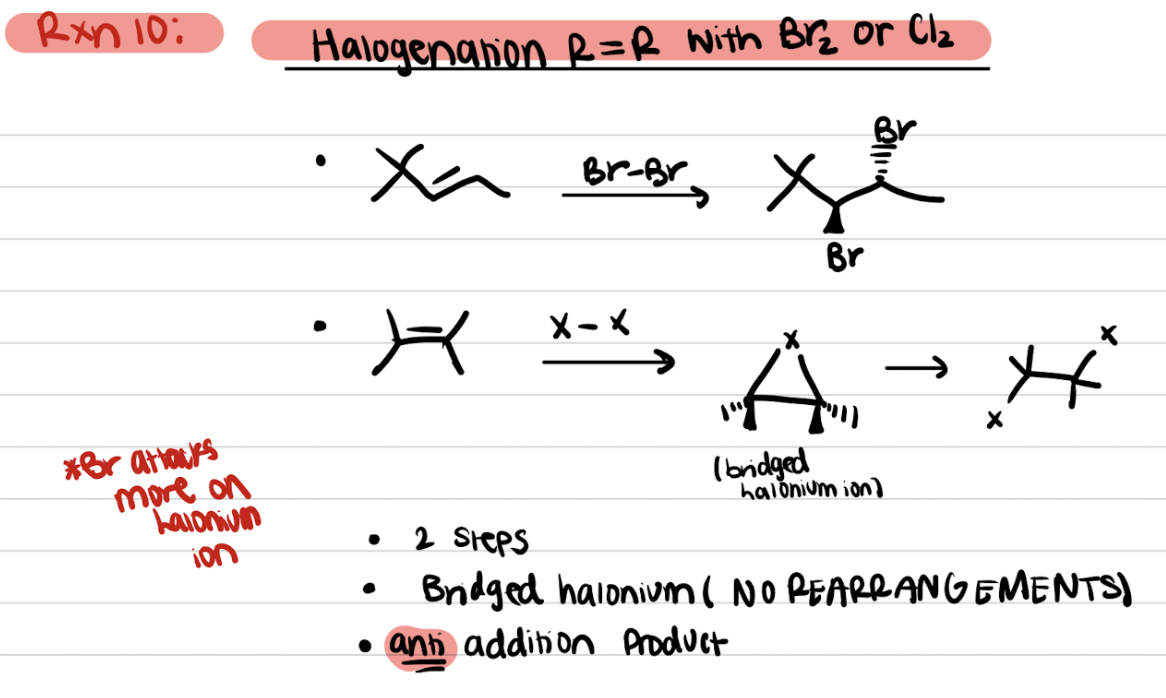
Addition of Alkenes - R=R reacts with Br2 or Cl2 and H2O or protic solvent (Halohydrin Formation)
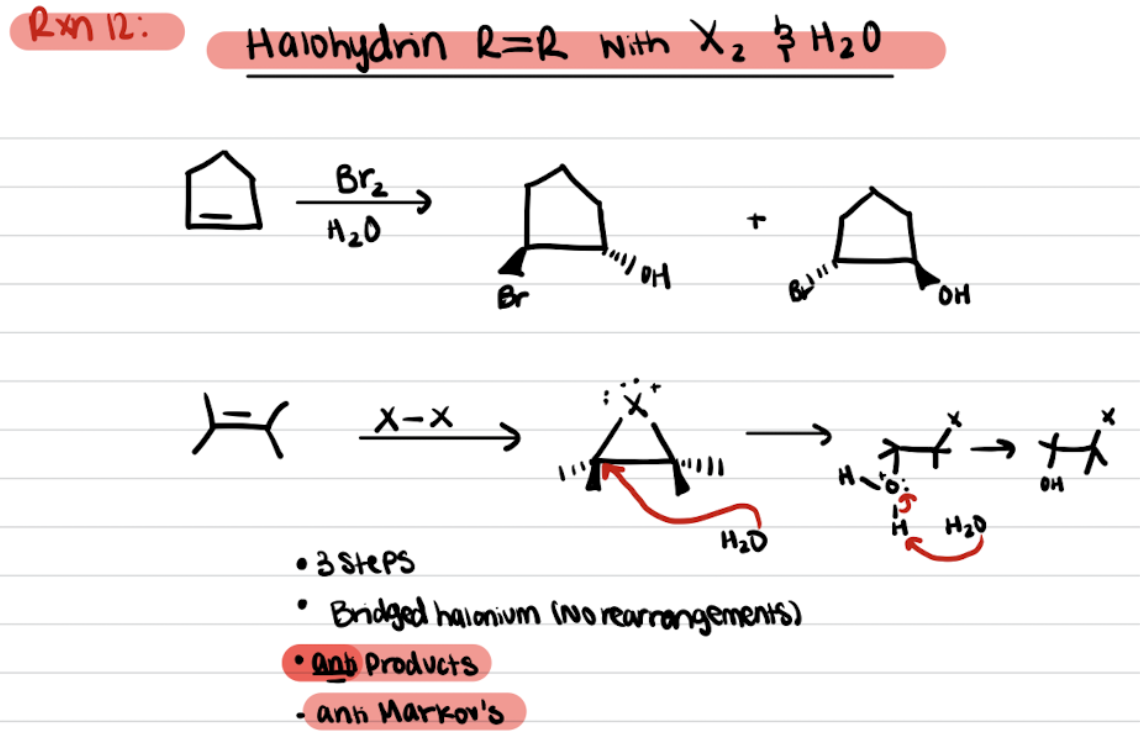
OH adds to the most substituted carbon, X added to the least substituted carbon
Anti products favored
Anti-Markovnikov’s addition
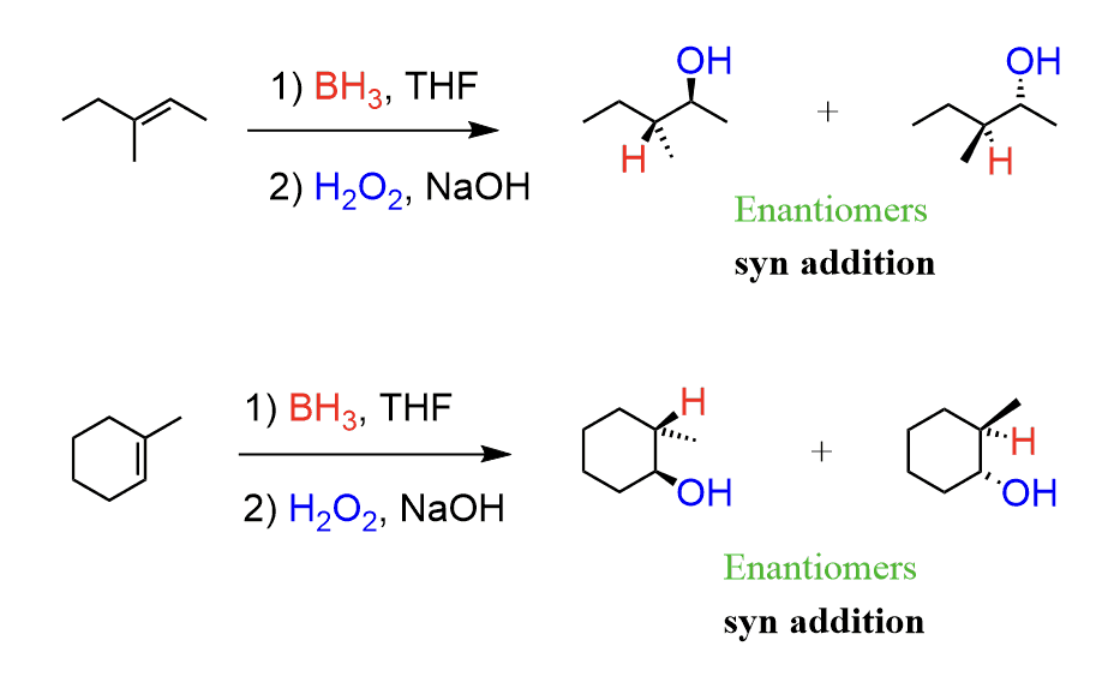
Addition of Alkenes - R=R with BH3 and H2O (Hydroboration/Oxidation)
Anti-Markovnikov’s Rule (H is added to the most substituted)
Adds an OH group to the LEAST substituted carbon in the double bond
Syn addition, the H and OH groups will be on the same plane (both dashed or solid)
2 steps - NO REARRANGEMENTS
BH3/THF followed by -OH, H2O2, H2O
B2H6/THF followed by -OH, H2O2, H2O
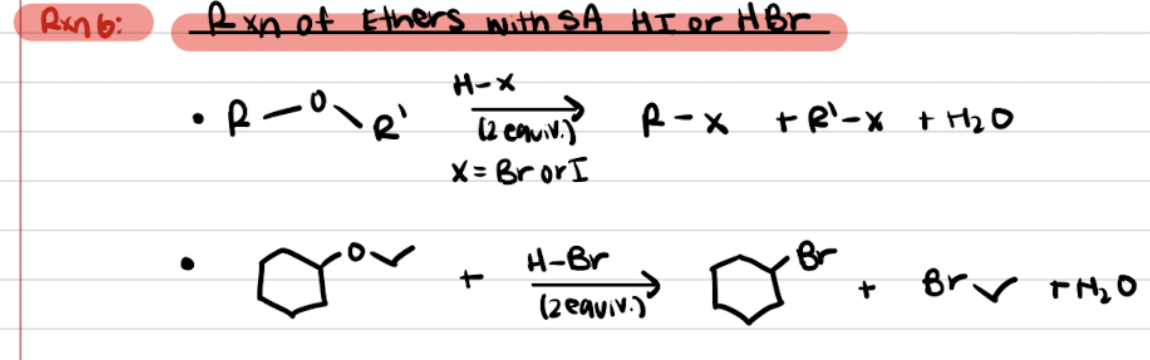
Reaction of Ethers with Strong Acids (HI or HBr)
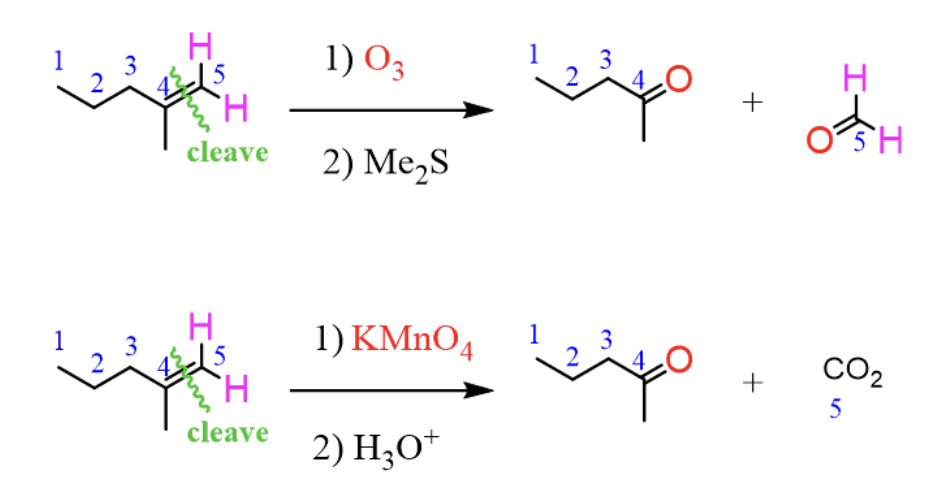
Ozonolysis of Alkenes (R=R with O3/KMnO4 in Me2S or H3O+)
Ozonolysis of alkenes produces a ketone and aldehyde with O3 and Me2S
and with KMnO4 and H3O+ in produces CO2
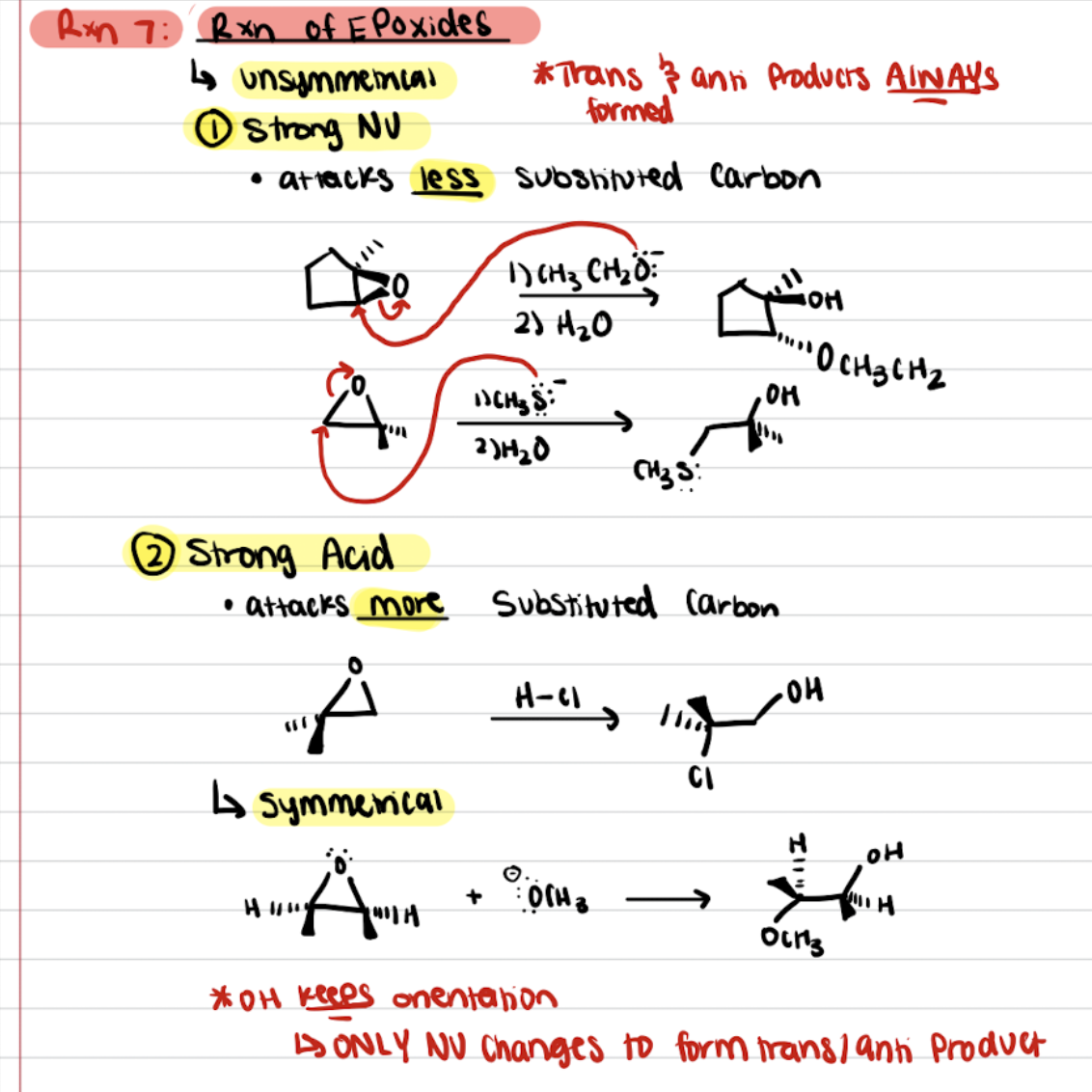
Reaction of Epoxides
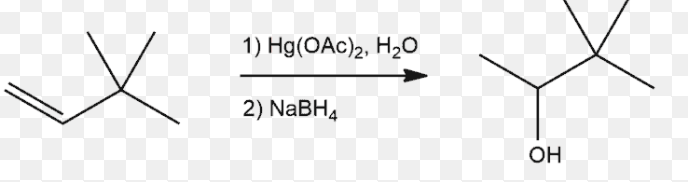
Alkoxymercuration Demercuration
No Carbocation Rearrangements
Markov’s Rule - OH on more substituted carbon and H on the less
1) Hg(OAc)2, H2O
2) NaBH4

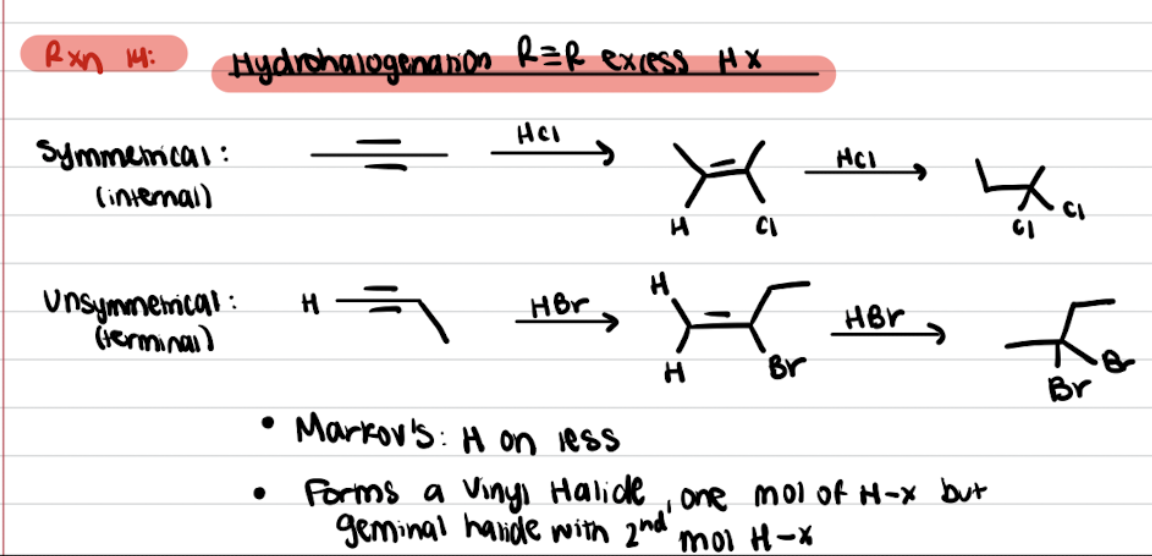
Addition of Alkynes R≡R excess HX (Hydrohalogenation)
Addition of Alkynes - R≡R with Excess X2 (Halogenation)
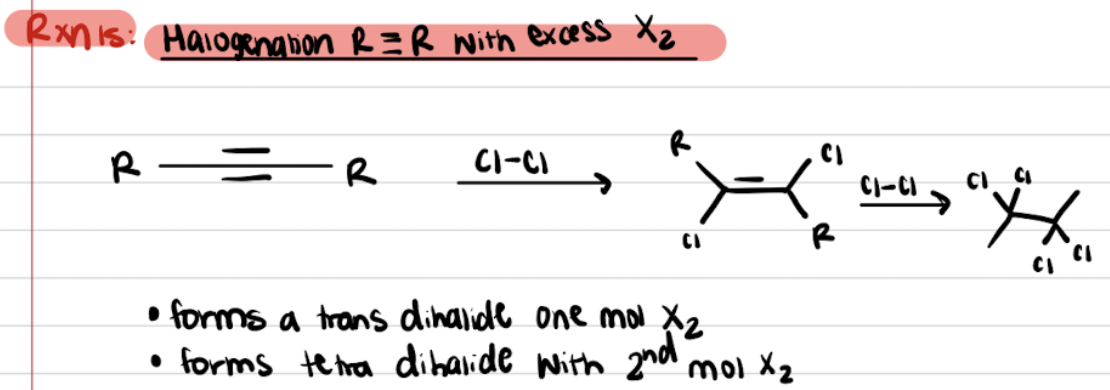
Addition of Alkynes - R≡R with H2O, H2SO4, and HgSO4 (Halogenation)
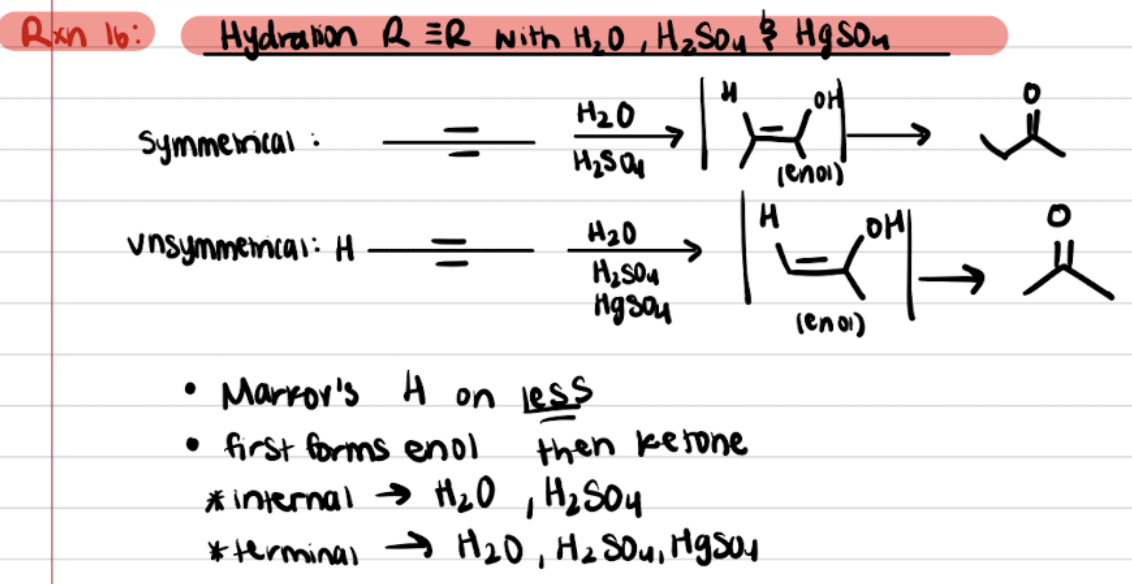
Addition of Alkynes - R≡R with BH3, then H2O2 and OH- (Hydroboration Oxidation)
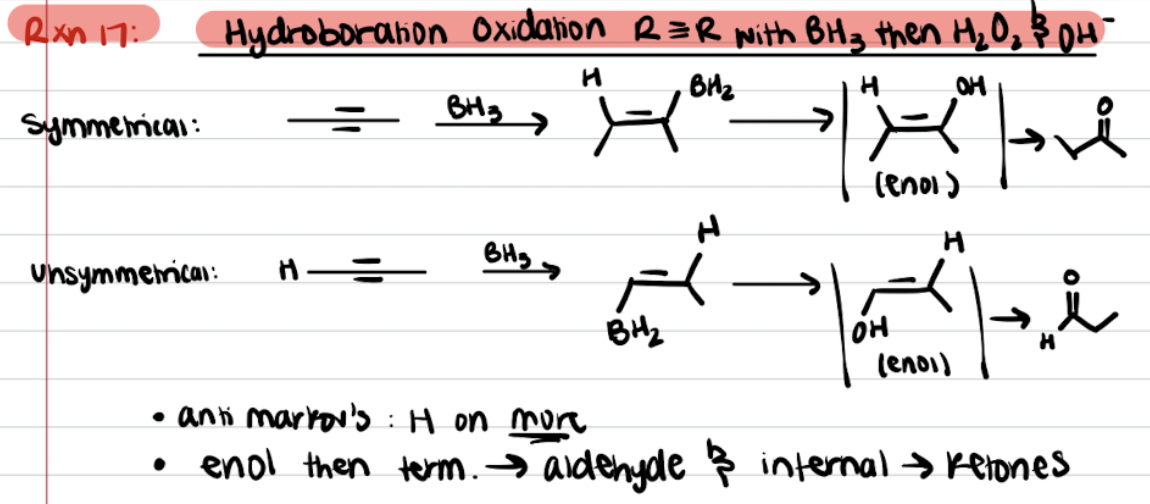
Reductive Ozonolysis
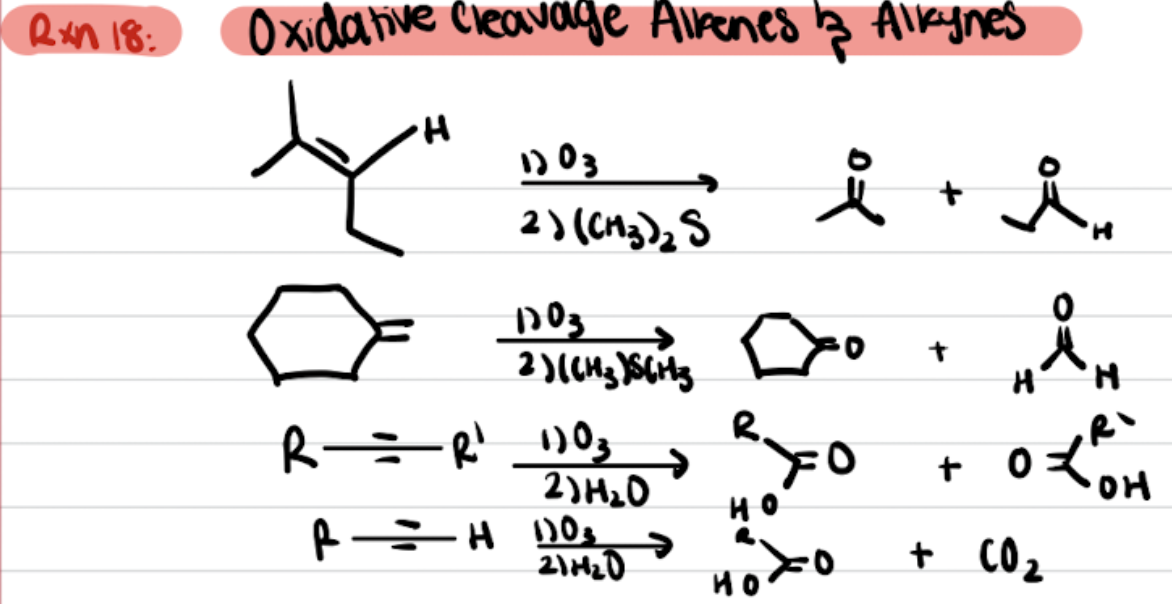
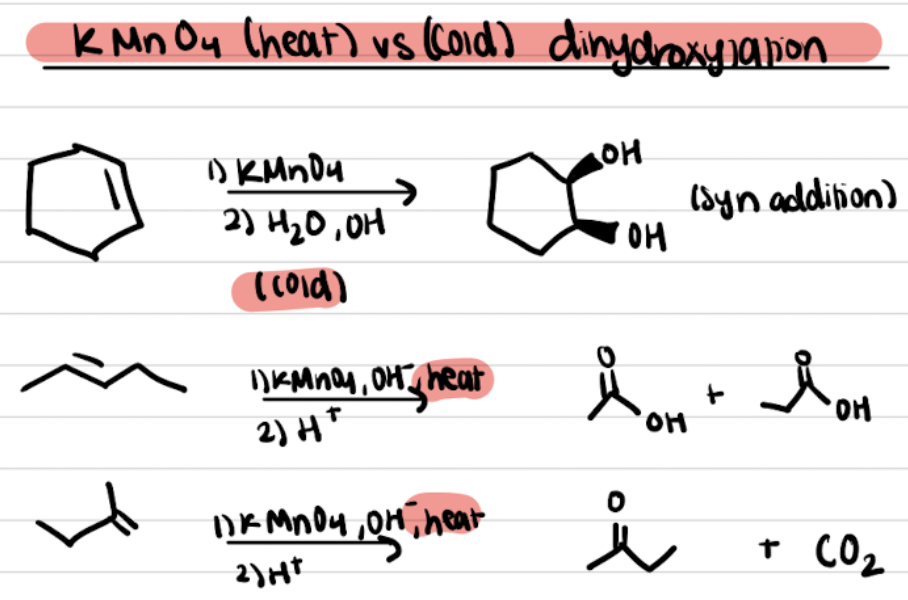
KMnO4 (heat vs cold) Dihydroxylation
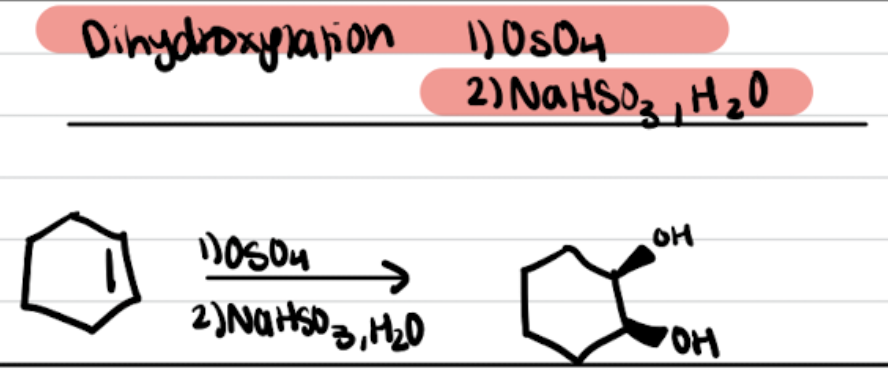
Dihydroxylation OsO4 and NaHSO3, H2O
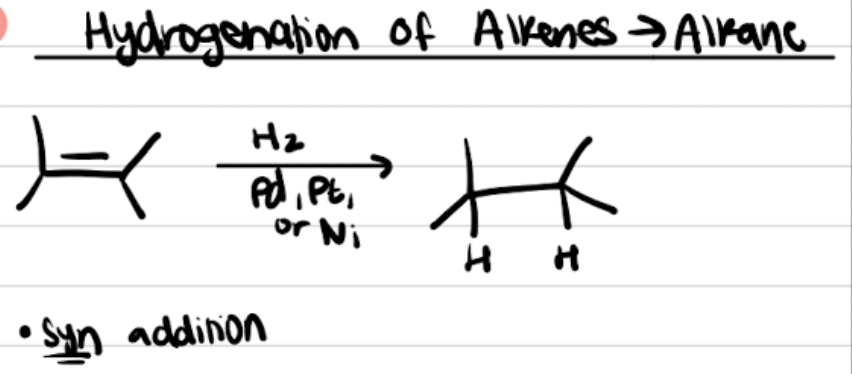
Hydrogenation of Alkenes to Alkanes
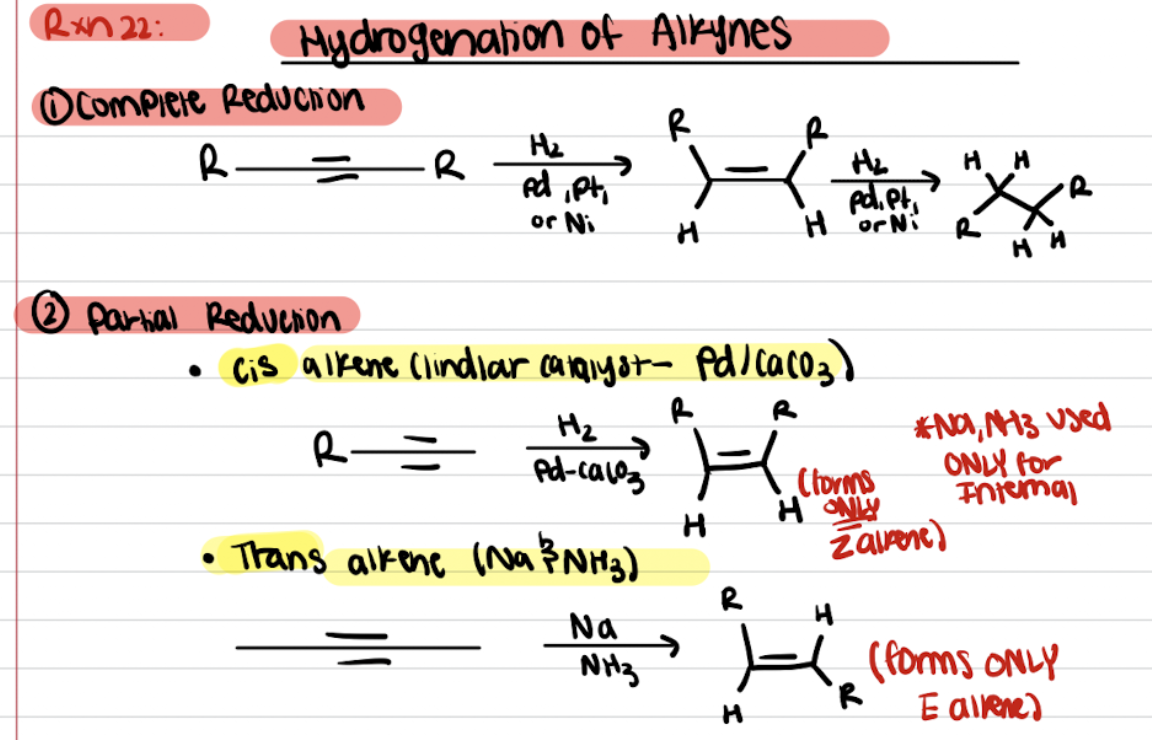
Hydrogenation of Alkynes
Regioselectivity vs Stereospecificity
Regioselectivity: where does it add (Markov’s Rule)
Stereospecificity: how does it add in 3D space (Syn or anti addition)
Hydrogenation of Aldehydes and Ketones
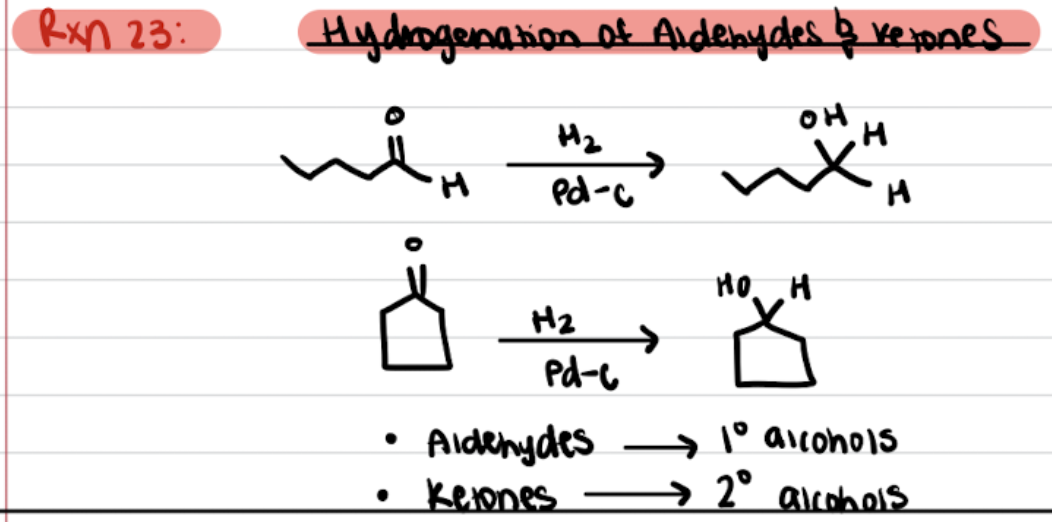
Chapter 9: Spectroscopy
Mass Spec - Molecular Ion Peak
Mass Weight of the Molecule
Mass Spec - Major Fragment
Find the the Functional Group and Cleave
Calculate the weight
HNMR - Number of H signals
the number of identical H’s in a molecule
Only count the H’s on Carbon
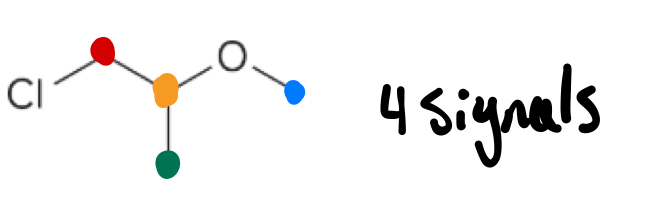
Down field vs Up field
Down field = left (more electronegative)
Up field = right (more electronegative)
Higher chemical shift = more downfield
more substituted or more electronegative atoms
When looking at the HNMR
Use n+1 to determine the splitting patterns
when looking at graph do n-1 to determine number of hydrogens in molecule
NO, OH and NH splitting = ALWAYS singlets
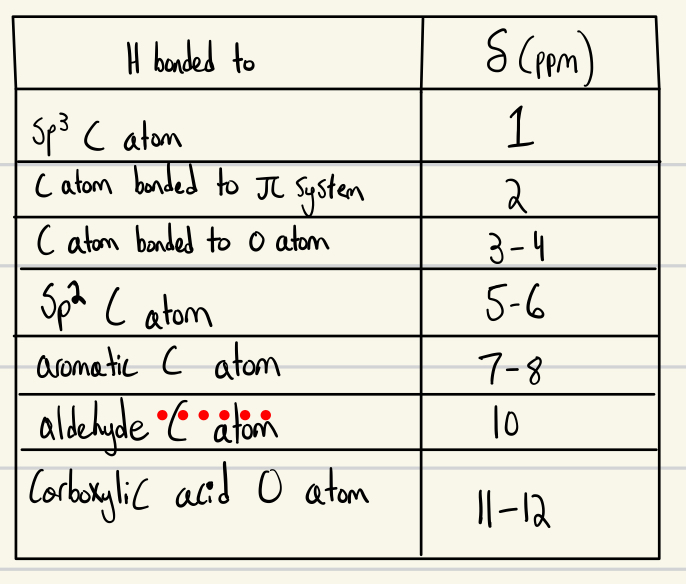
H NMR PPM - Chemical Shift
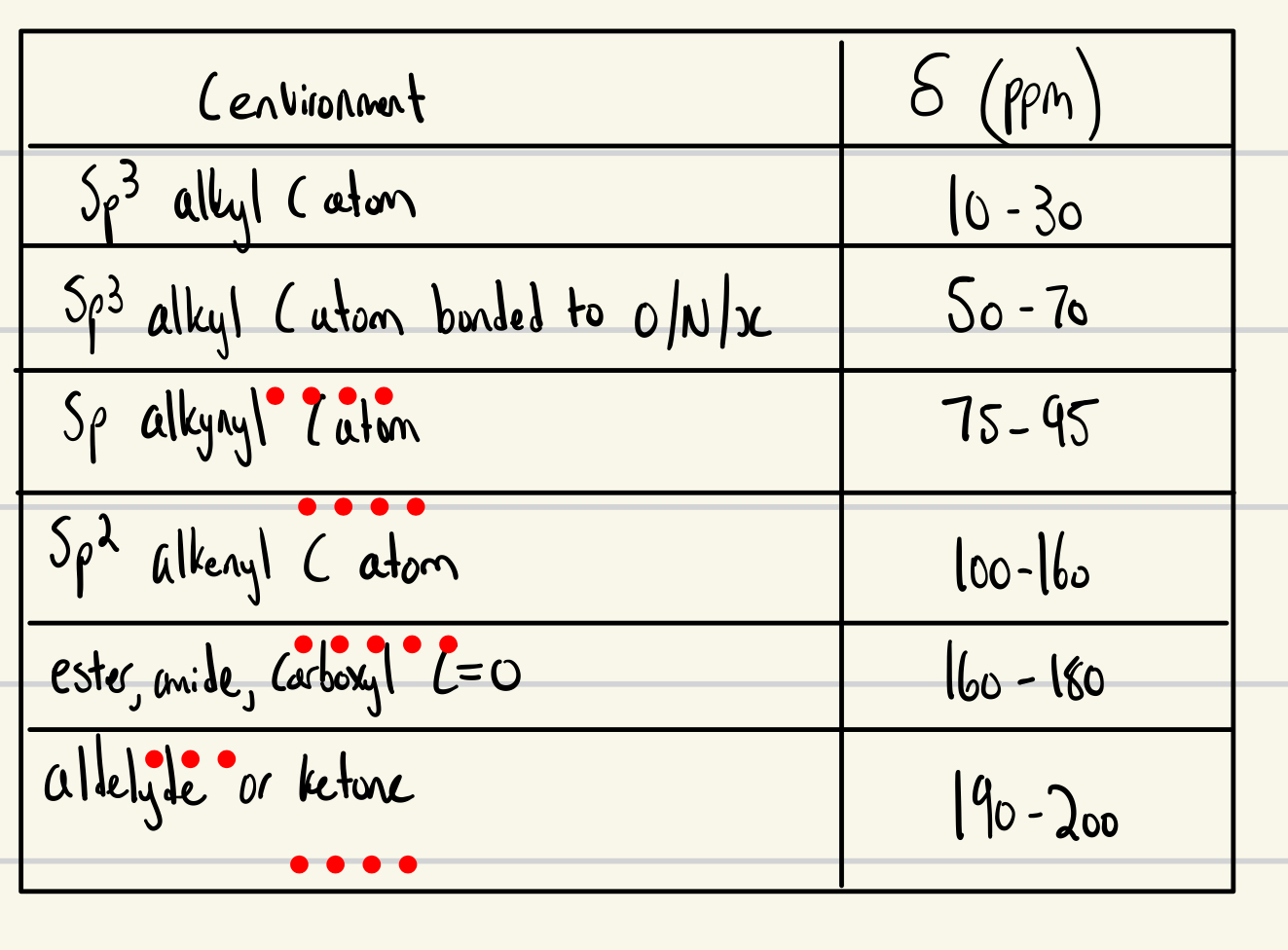
C NMR PPM - chart
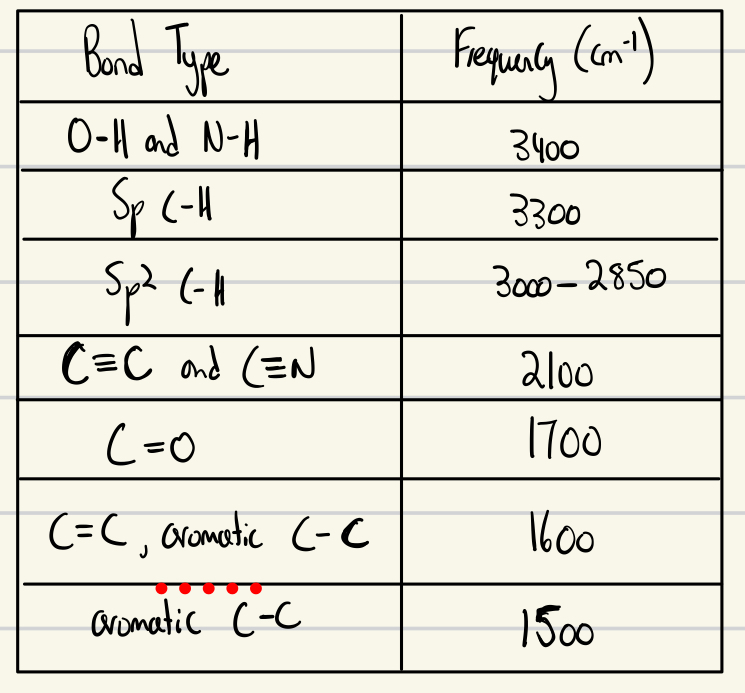
IR Spectroscopy chart
Chapter 8: Addition Reactions to Alcohols and Ethers
Catalytic Hydrogenation of Aldehydes and Ketones
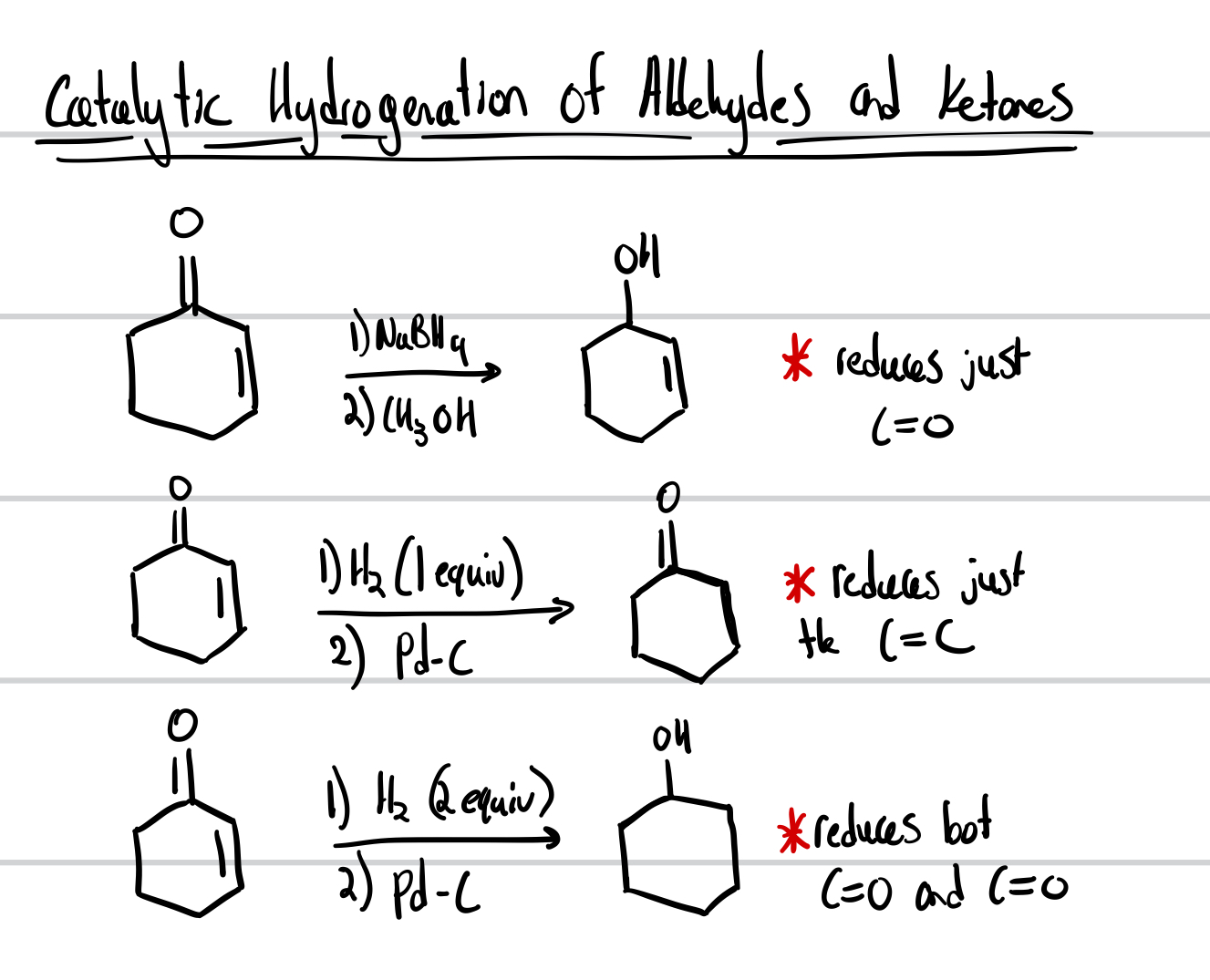
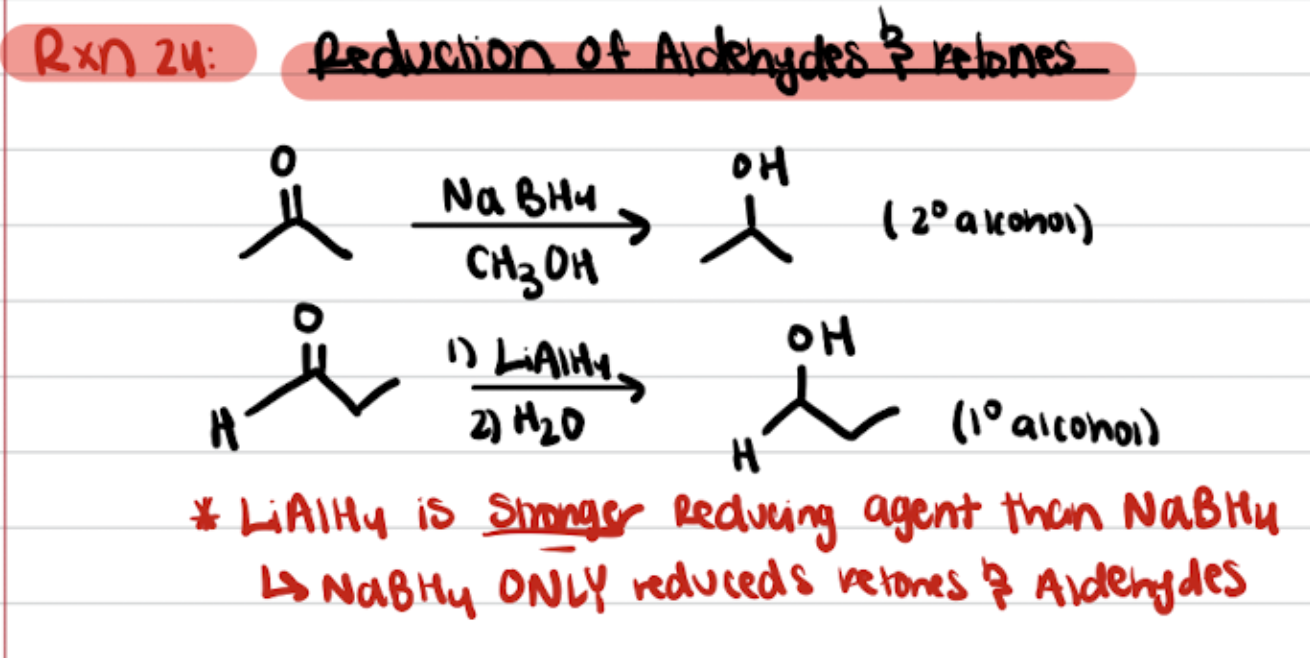
Reduction of Aldehydes and Ketones
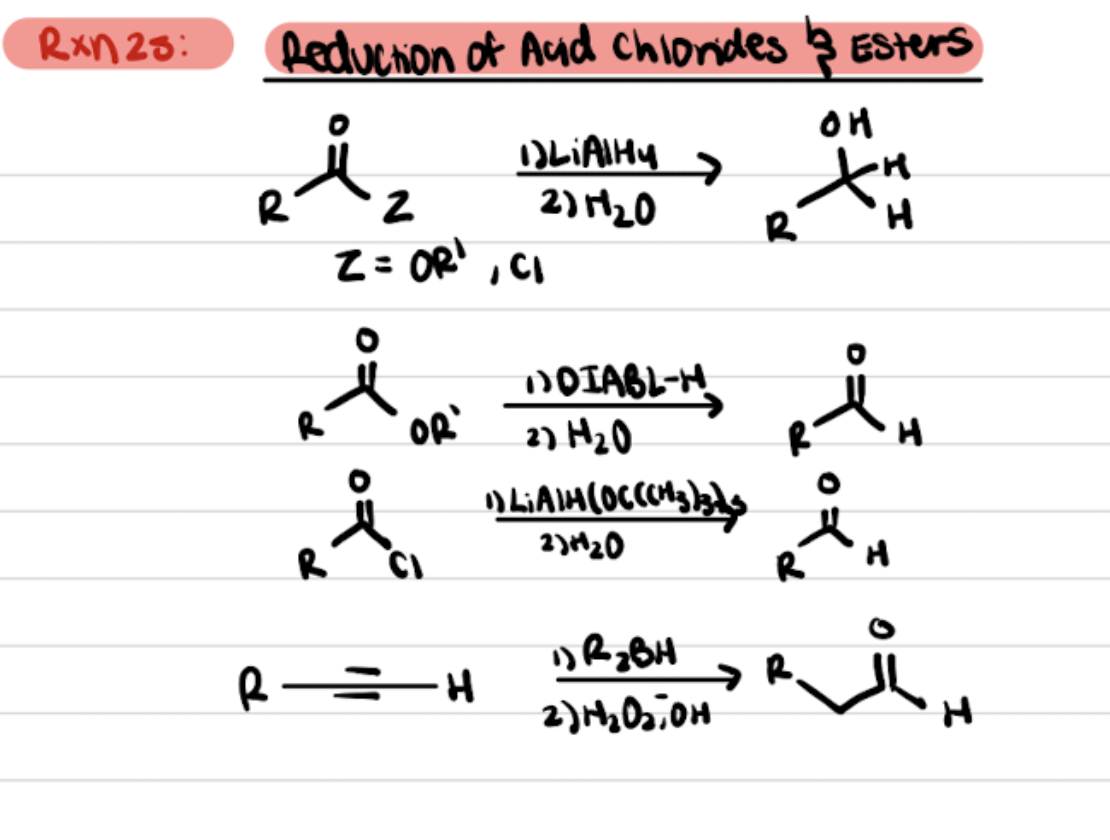
Reduction of Acid Chlorides and Esters
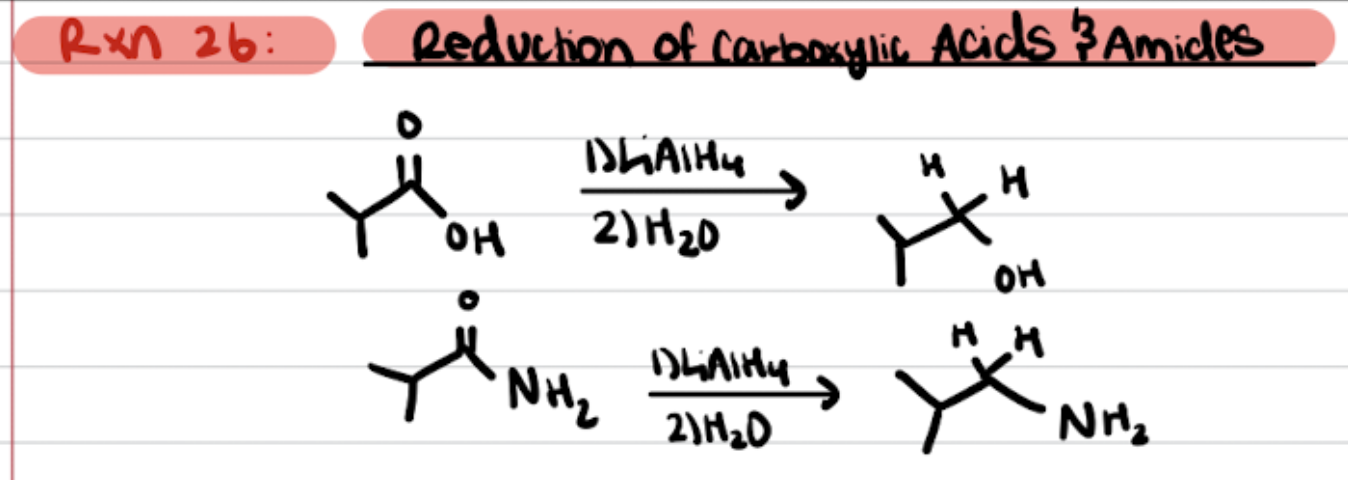
Reduction of Carboxylic acids and Amides
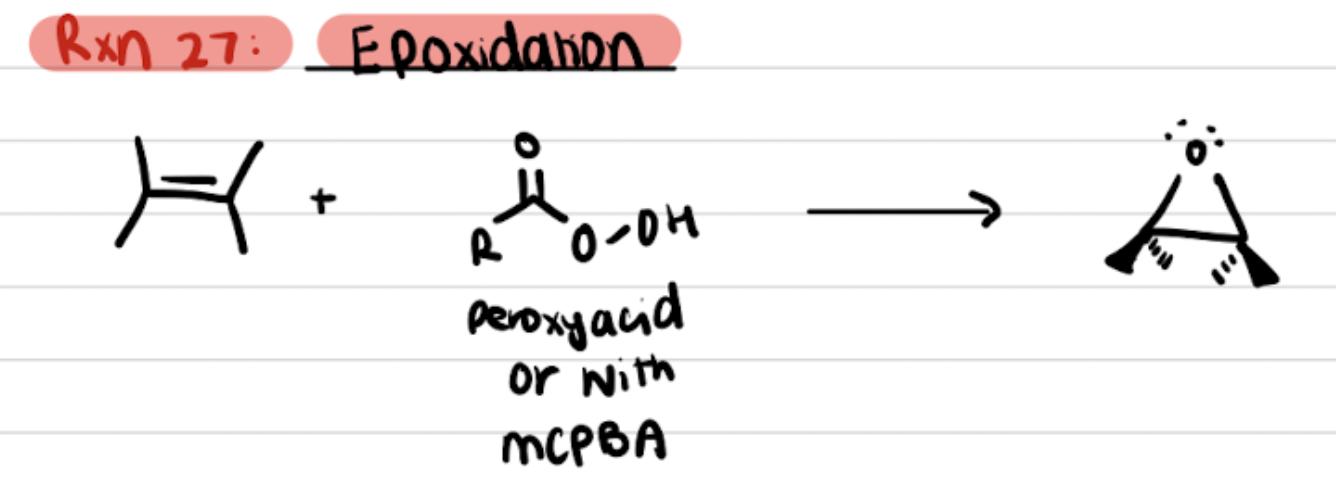
Epoxidation
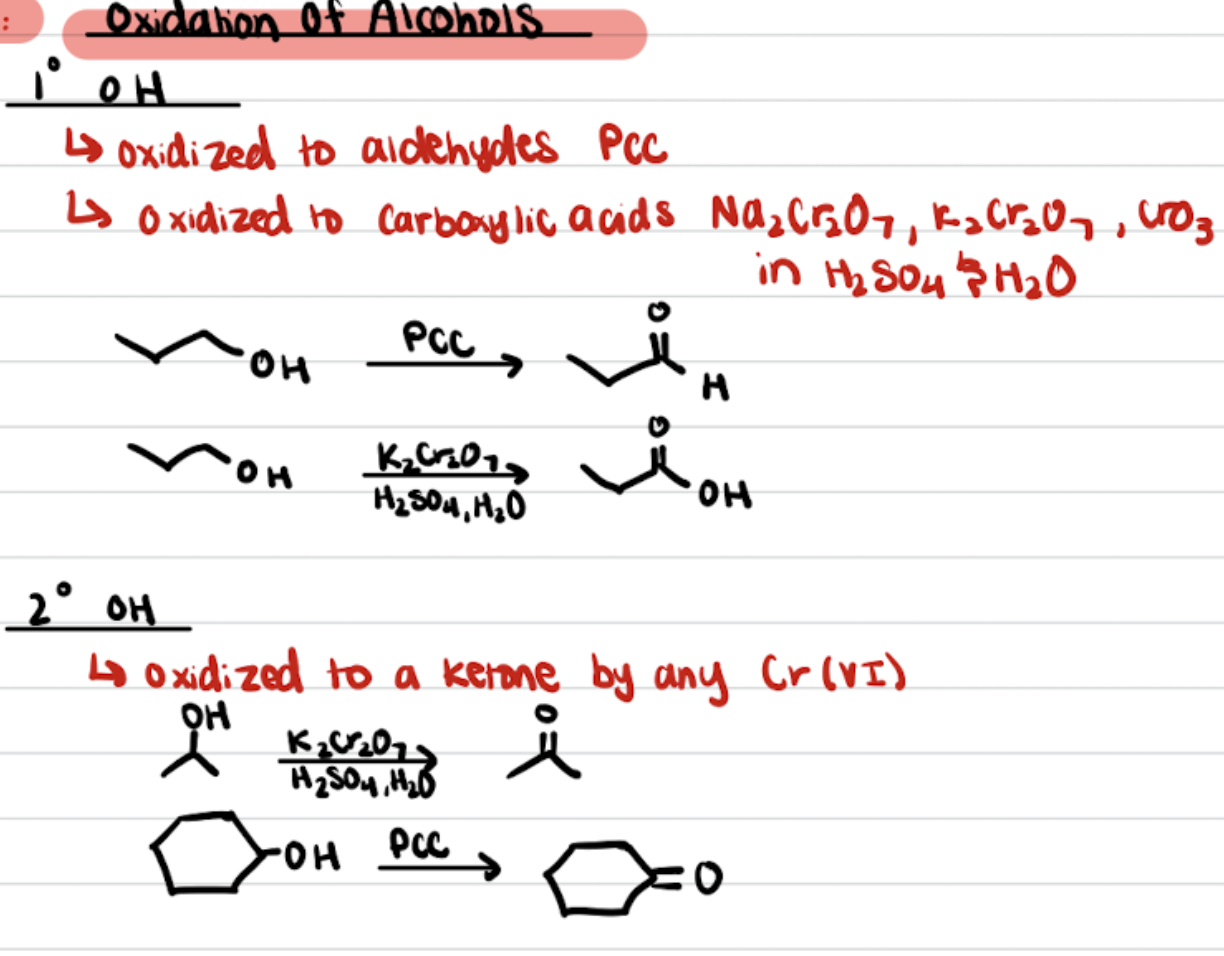
Oxidation of Alcohols
Protecting OH and NH groups
