Ionization Energy
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
What is ionization energy?
The energy required to remove an electron from a neutral atom or cation in the gaseous phase.
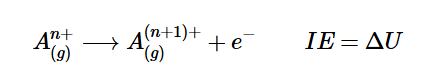
What are I₁, I₂, and I₃ in ionization energy?
I₁: First ionization energy — removing an electron from a neutral atom.
I₂: Second ionization energy — removing an electron from a +1 cation.
I₃: Third ionization energy — removing an electron from a +2 cation.
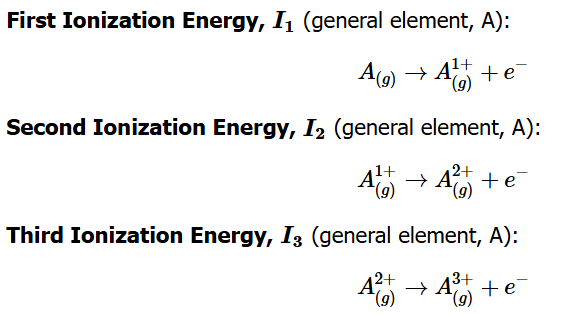
How do ionization energies change as electrons are removed (I₁ → I₂ → I₃)?
Each successive ionization energy is larger than the previous:
I1 < I2 < I3
This is because removing an electron from an increasingly positive ion requires more energy
What does a higher ionization energy indicate?
A stronger attraction between the nucleus and the electron, meaning more energy is needed to remove that electron.
What is the general trend for ionization energy across a period?
Ionization energy increases as you move across a period because Z* increases, strengthening the nuclear pull on electrons.
What are key exceptions to the ionization energy trend across a period?
Ionization energy is lower than expected when:
Removing an electron empties a p-subshell.
Removing an electron results in a half-filled p- or d-subshell, which is relatively stable.
For d- and f-block elements, IE increases more gradually since d- and f-electrons are weakly penetrating and experience lower Z*.
What is the trend for ionization energy when moving from one period to the next (down a group)?
Ionization energy drops significantly at the start of a new period.
This is because removing an electron from the new outer s-subshell requires less energy; the electron is farther from the nucleus and more shielded.