The Periodic Table - Chemistry Topic 2
1/12
Earn XP
Description and Tags
Chemistry Topic 3, actually, on the Periodic Table
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
Atomic Theory
An atom is the smallest particle of an element. If a substance is made up of all the same type of atom, then it is called an element. For example, hydrogen is made up of only hydrogen atoms, so we call it an atom.
Arrangement of the Table
The Periodic Table groups all the elements into groups (vertical columns) and periods (horizontal rows).
A Lithium atom diagram
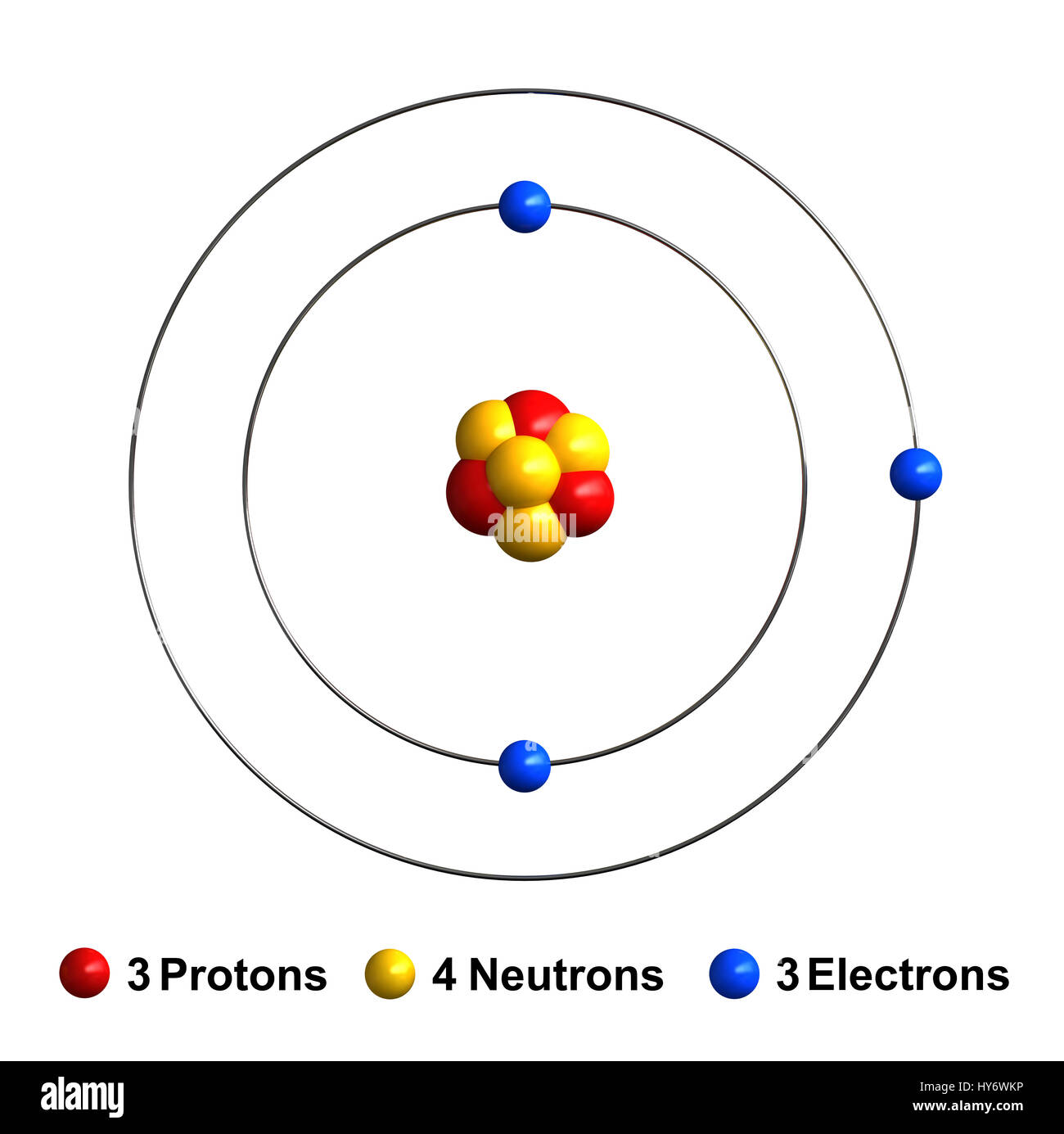
Protons have a positive charge and neutrons have negative charge. Neutrons have a neutral charge.
How it works
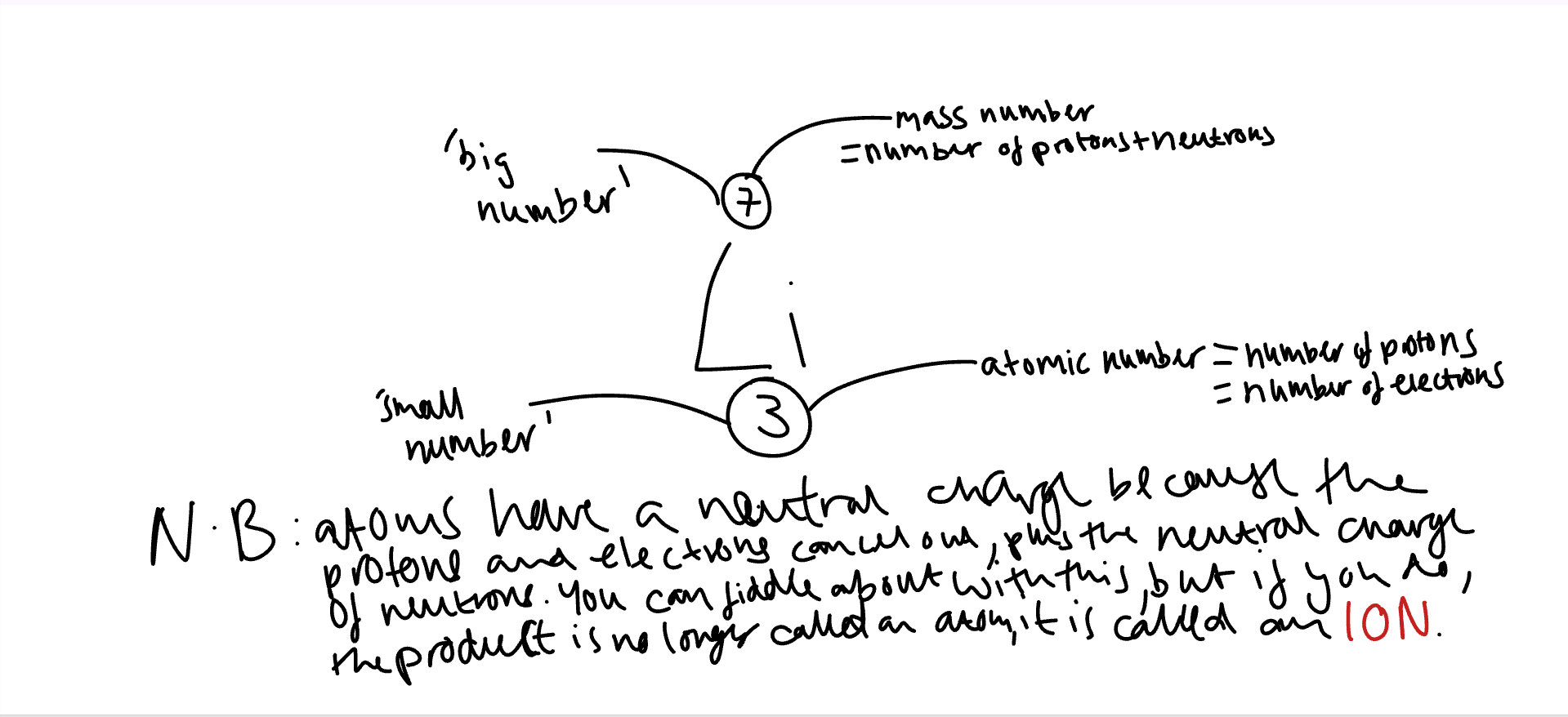
How do scientists communicate?
In order for scientists all over the world to communicate clearly, it was decided that each type of atom should have a universal chemical symbol. Otherwise if a Spanish chemist was trying to speak to an English chemist, one would be talking about ‘oro’ and the other would be talking about ‘gold’. As it is, they are both talking about ‘Au’.
How to write element formulae?
the first letter is always capital (e.g: you would never have oxygen, you would always have Oxygen)
If there is one, the second letter is always lowercase (e.g: Beryllium as Be)
What is a compound?
A compound is a substance that is made up of more than one type of atom or element, and that all atoms and elements are chemically bonded.
How to name compounds?
The element furthest to the left of the Periodic Table comes first in the name
The second element changes the end of its name to -ide
If there are three elements and one of them is oxygen, the second element in the name changes the end of its name to -ate.
If there are two or more elements in the same column, the one on the bottom row comes first in the name.
Chemical Properties:
how they burn
pH score
electrical conductivity
how it bonds with other elements and compounds
will it effervesce if in contact with an acid?
reactivity
toxicity
flammability
Physical Properties
appearance
melting and boiling point
viscosity
whether it can be compressed
state at room temperature
starch content
heat conductibility
malleability
sonorous?
radioactivity
volume/mass/density
electrons, protons, neutrons
atomic value
colour
length
opacity
brittle?
What is a mixture?
A mixture is 2 or more elements an/or compounds that are NOT chemically bonded together.
COMPOUNDS vs MIXTURES
Compounds, Mixtures:
They contain at least two substances
They show the properties of their component elements or compounds
The component elements or compounds need not to be in fixed proportions
No chemical change takes place when making a mixture - no new substance is formed
No or very little energy change takes place when they are formed
They are single substances
They do not show the properties of their component elements
The component elements are in fixed proportions
They are formed by means of a chemical change - a new substance is formed
An energy change takes place when they are formed (heat is frequently given out)
True or false?
All compounds are on the Periodic Table - F
Elements are made up of only one type of atom - T
All solids are elements - F
All molecules are compounds - F