Chapter 9: carbohydrates
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
82 Terms
Functions of carbohydrates
metabolism, storage and generation of energy, molecule recognition, cellular protection, cell adhesion, biological lubrication, and maintenance of biological structure
Carbohydrate formula
(CH2O)n
When is a carbohydrate not a saccharide?
when n= 2 or 1
Saccharides
carbohydrates of n>3 and their derivatives (contain amino, sulfate or phosphate groups)
Sugar
saccharides that do not have modifications
Monosaccharide
simple sugars and derivatives with 3 to 9 C atoms
Oligosaccharide
compound from by linking several monosaccharides together (eg disaccharides that are two monosaccharides linked together)
Polysaccharide
polymer from multiple saccharide units. May be homopolysaccharide or heteropolysaccharide.
Glycan
generic term for oligosaccharides and polysaccharides
Formaldehyde
carbohydrate of n=1. Not a sugar, poisonous
Acetaldehyde
carbohydrate of n=2. Not a sugar, toxic
Carbohydrates of n= ? gives compounds with properties of sugars
3-9
Aldose vs ketose
aldoses contain aldehyde functional groups while ketose contain ketones

Enantiomers
optical isomers that are are mirror images
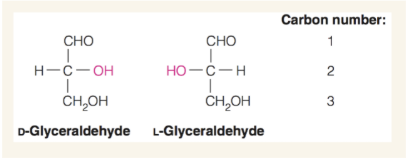
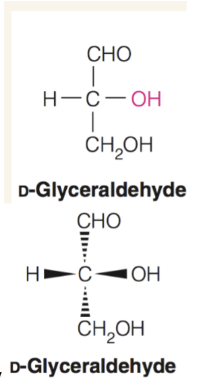
How to identify L vs D isoform
Use the direction of the −OH group on the chiral carbon furthest from the carbonyl group to determine which isomer it is. On a Fischer projection, it is the L isomer if the −OH group points left and the D isomer if it points right.

What is the 1 carbon of an aldose?
the C in the CHO group (aka where the aldehyde group is attached)
How does a fischer projection show stereochemistry
horizontal bonds point towards viewer and vertical bonds point away
How to identify a chiral carbon?
carbon must have 4 unique functional groups attached to it
Diastereomers
optical isomers that are NOT mirror images of one another
All life forms only use —- - amino acids and — - sugars
L -amino acids and D-sugars
What may be an explanation for D-sugars being more common in nature than L-sugars?
higher ring stability
What may be an explanation for L-amino acids being more common in nature than D-amino acids?
preference in terms of enzyme evolution
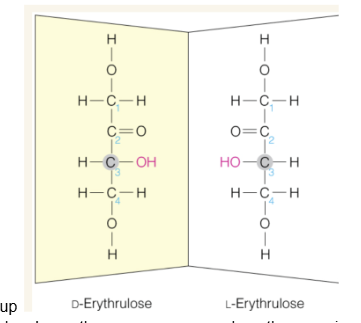
Ketoses always have —- (more or less) chiral carbons than aldoses
1 less chiral carbon

what is the stereochemical relationship between D-threose and D-Erythorse? What is the stereochemical relationship between D-threose and L-Threose? Between L-Thresose and D-erythrose?
diastereomers; enantiomers; diastereomers
What is the 1 carbon of ketoses
the CH2OH group on the end of the sugar closest to the ketone group
The more chiral carbons, the more —- a sugar has
the more isomers
Which one has more isomers, a five carbon aldose or a five carbon ketose and why?
the aldose because aldoses usually have more chiral centers than ketoses of the same carbon number
Show the reaction for hemiacetal formation. What is notable about the product of this reaction?
The carbon now has four different substituents so it becomes a chiral center

5 or 6 carbon sugars may undergo —-- in order to form a ring
hemiacetal formation
Furanose
5 membered ring formation of a sugar
Pyranose
6 membered ring formation of a sugar
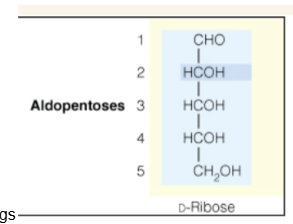
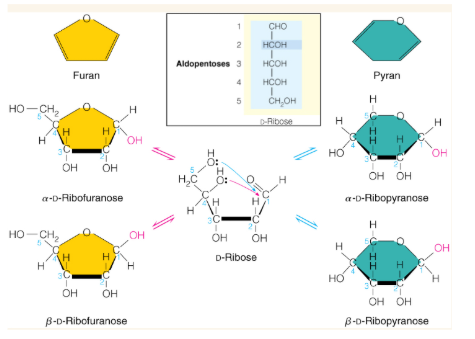
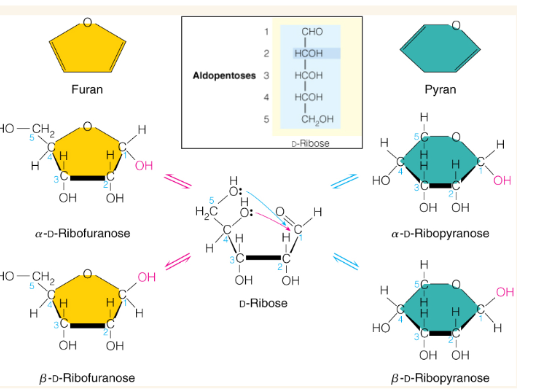
Show how this sugar may cyclize into rings
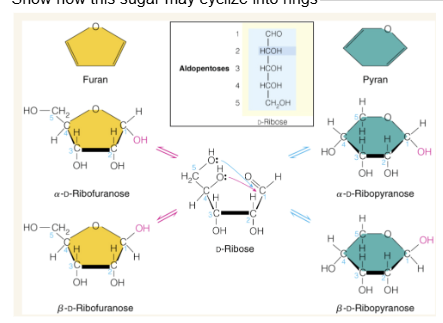
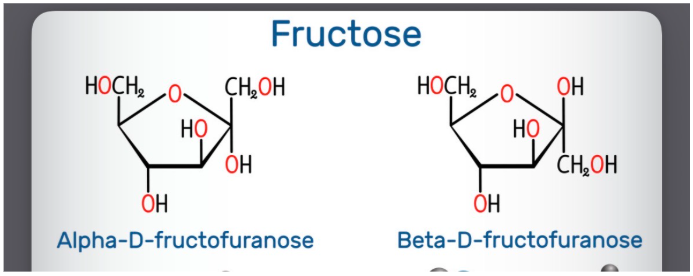
Alpha vs beta form of sugar rings
cyclization creates a new asymmetric center called the anomeric center. Beta form has an anomeric center with the -OH group pointing up while alpha has the -OH group pointing down
Anomeric center
new asymmetric center created by ring formation of sugar. May be designated as alpha or beta
Mutarotation
the process where cyclic sugars, like glucose, change their optical rotation as their anomeric forms alpha and beta interconvert in solution until they reach an equilibrium mixture. This occurs because the cyclic form can open into a straight-chain form and then re-close into either the alpha or beta cyclic structure
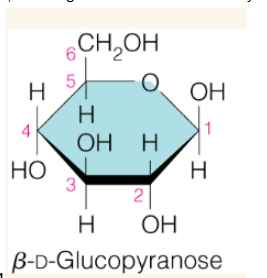
Haworth projection
a way to draw cyclic sugar molecules (carbohydrates) as flat, ring-like structures, showing their 3D stereochemistry with groups pointing up or down relative to the ring
Epimer
stereoisomers differing in configuration about one carbon (not the anomeric carbon)
Anomer
stereoisomer that differs in configuration at at the anomeric carbon
Alpha vs beta cyclic structure of sugars
the alpha structure occurs when the -OH attached to the anomeric carbon is pointing down while the beta conformation has it pointing up
For pyranoses, when O is away from the viewer and the hemiacetal group is at the right side, how can you identify if the sugar is L or D and alpha or beta
CH2OH group above the plane=D or CH2OH group below the plane=L; C1 –OH above the plane=β or C1 –OH below the plane=α
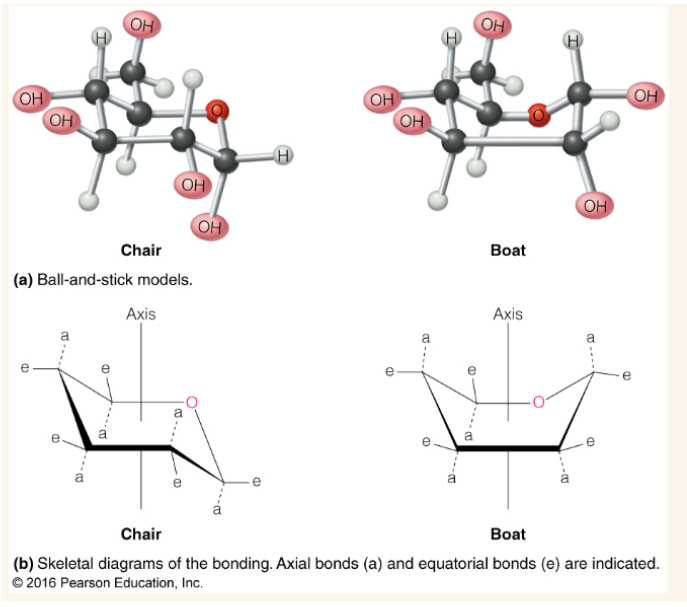
Chair vs boat form of pyranoses (which is more stable)
chair form is lower energy and therefore more stable
t/f all stereoisomers have the same chemical formula
true
Sugar phosphates
important intermediates in metabolism. Functions as activated compounds in syntheses
Alditol
result of reduction of the sugar carbonyl. Sometimes creates inner molecular symmetry. These sugar alcohols are used widely in the food industry as thickeners and sweeteners. Does not cause tooth decay and arent absorbed well to small intestine.
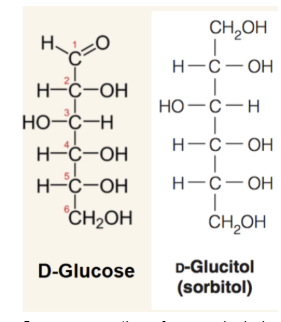
Overconsumption of sugar alcohols can lead to what?
bloating, diarrhea and flatulence
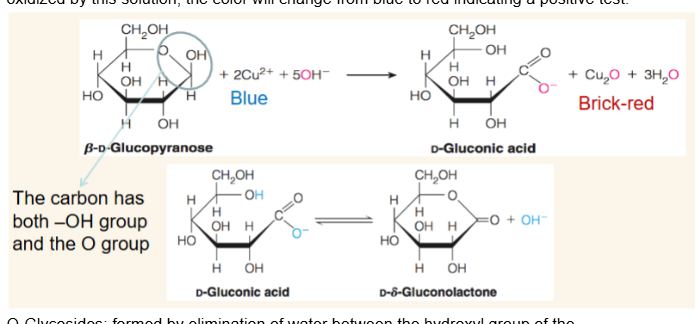
Aldonic acid
result of oxidation of C1 of a sugar.
Uronic acid
result of oxidation of C6 of a sugar
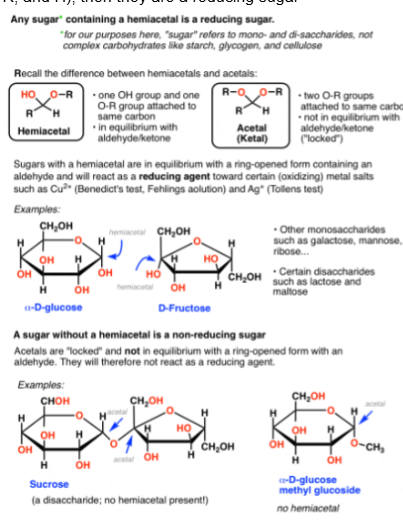
How to experimentally test for reducing sugars?
The solution of Cu(II) (Fehling’s/Benedict’s/Tollen’s) may be used as an analytical test for reducing sugars. If the sugar’s reducing end is oxidized by this solution, the color will change from blue to red indicating a positive test.
O-Glycosides
formed by elimination of water between the hydroxyl group of the anomeric carbon of a cyclic saccharide and the hydroxyl group of another compound. Newly formed bond is called a glycosidic bond
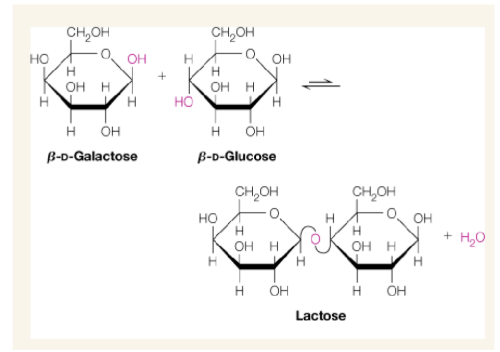
Glycosidic bond formation
bond formed by elimination of water between hydroxyl group of anomeric carbon of a cyclic sugar and hydroxyl group of another compound

Reducing end in glycosidic bond formation
here in the example, the reducing end of Galactose interacts with C4 of Glucose to form lactose and eliminate one water
Monsaccharides can form polysaccharide via —- bonds
glycosidic
Maltose (structure and abbreviation)
𝛼-D-Glcp-(1->4)𝛼-D-Glcp

Sucrose(structure and abbreviation)
𝛼-D-Glcp(1->2)𝛽-D-Fruf

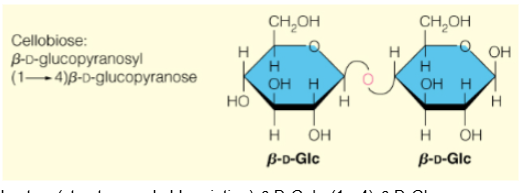
Cellobiose(structure and abbreviation)
𝛽-D-Glcp-(1->4)-𝛽-D-Glcp
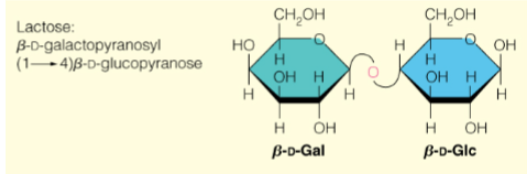
Lactose(structure and abbreviation)
𝛽-D-Galp-(1->4)-𝛽-D-Glcp
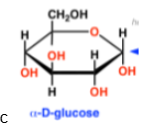
alpha-D-glucose(structure and abbreviation)
Glc
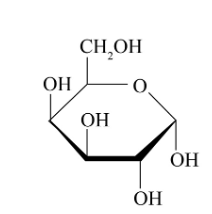
alpha-D-galactose(structure and abbreviation)
Gal
alpha-D-fructose(structure and abbreviation)
Fru
When drawing disaccharides, how should it be oriented?
reducing end (if there is one) must be on the right
What polysaccharides may not be oxidized by Fehling’s solution?
polysaccharides which do not contain a reducing end (example; sucrose)
How to tell if a polysaccharide has a reducing end or not based on chemical structure
check the anomeric carbon of the monosaccharide subunit not involved in a glycosidic bond. If there is an -OH attached, that sugar is reducing. Otherwise it is not. Other method is to check if the sugar has a hemiacetal group (Carbon attached to a OH, OR, R, and H), then they are a reducing sugar
5 major features of disaccharides
sugar monomers involved and their stereochemistry, carbons involved in the linkage, order of the sugars (note free anomeric carbon can undergo oxidation), configuration of the anomeric carbon (alpha or beta), and ring configuration (p or f) (p means 6 membered ring and f means 5 membered)
If there is a glycosidic bond between carbon – and carbon — of two monosaccharides, that disaccharide will not have a reducing end. Why?
1 and 1; there will be no anomeric carbon with an -OH group
Starch
storage polysaccharide of plants. Contains both amylopectin and amylose
Amylopectin
alpha (1->4) glucose polymer with a (1->6) branches. Part of starch.

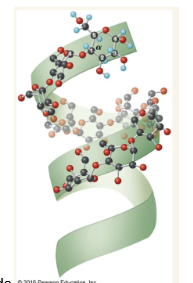
Amylose
alpha (1->4) unbranched polymer. Part of starch. Forms into a helix with a large interior core and stabilized by H-bonds
Glycogen
storage polysaccharide of animals and microbes. Like amylopectin but higher MW with shorter and more frequent branch points
Sticky rice has more —- in its starch, making it sweeter
more branches in the amylopectin
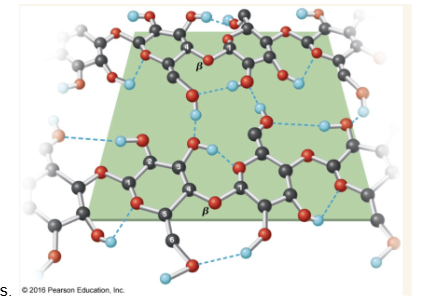
Cellulose
major structural polysaccharide in plants. Linear glucose polymer with beta(1->4) linkages.
Xylans and glucomannans
modified polysaccharides that are found in fibrous parts of plants
How is the cell wall of plant cells organized
cellulose chains form microfibrils which form the plant cell wall
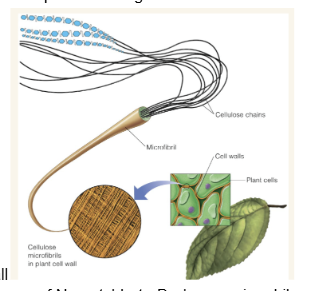
Chitin
homopolymer of N-acetyl-beta-D-glucosamine. Like a derivatized form of cellulose. Major structural component of the exoskeleton of arthropods and mollusks. Comparable to collagen in providing a matrix for mineralization

Glycosaminoglycans
serve structural and nonstructural roles in vertebrates.
Gram positive vs gram negative bacteria cell wall
gram negative bacteria has double layer of lipid membrane while gram positive bacteria has a thick peptidoglycan cell wall
Peptidoglycan cell wall
cell wall of gram positive bacteria.Cross-linked, multilayered polysaccharide-peptide complex. Site of action of the earliest antibiotics
Penicillin
strong antibiotic as it inhibits formation of the peptidoglycan cell wall
glycoproteins
proteins with attached carbohydrate chains that play crucial roles in cellular functions like cell recognition, immune response, and structural support
N-linked glycoprotein
saccharide bound to a protein sidechain via a Nitrogen found in the peptide side chain ( for example asparagine)
O-linked glycoprotein
saccharide bound to a protein sidechain via a Oxygen found in the peptide side chain ( for example threonine)
How do glycoproteins determine blood type
Glycoproteins on red blood cell surfaces act as antigens (A, B, or neither) that define your ABO blood type
Which of these polymers has a lower energy compared to its hydrolyzed (monomeric) state- DNA, RNA, polypeptides, and poly saccharides
none of these polymers are in a lower energy state than their respective monomer