psychopharmacology chapter 3
1/19
Earn XP
Description and Tags
quiz 1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
drug targets
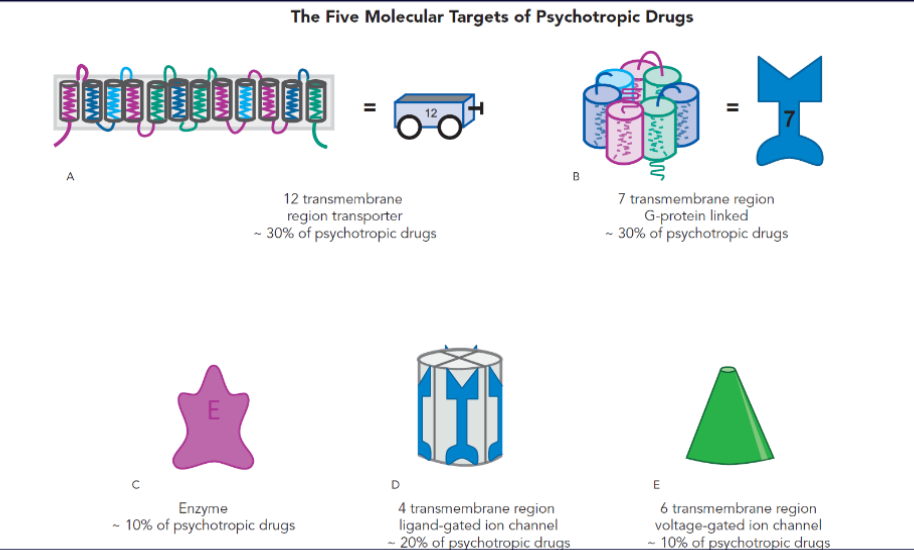
psychotropic drugs have many mechanisms of action, but they bind to specific molecular sites
1/3: target neurotransmitter transporters
1/3: target G-protein-coupled receptors
10% target enzymes
the rest target ion channels
location of transporters
they are found on neuronal membranes and glial cell membranes
membrane is semi-permeable, selective movement
maintains proper neurotransmitter levels and prepares for future neurotransmission
recapture/reuptake
transporter takes neurotransmitter back into the presynaptic neuron
transport into synaptic vesicle
storage and future release
structure of transporters
belongs to a superfamily of 12-transmembrane-region proteins
each transporter crosses the membrane 12 times
major subclasses of transporters
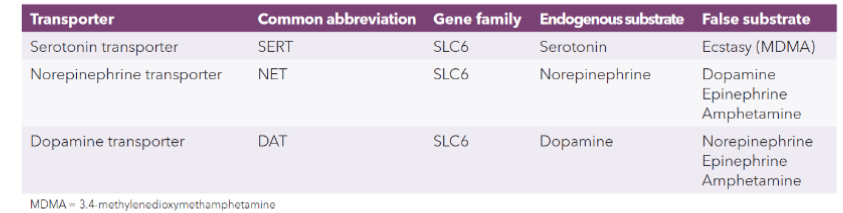
sodium/chloride-coupled transporters (SLC6 family)
glutamate transporters (SLC1 family)
sodium/chloride-coupled transporters (SLC6)
handles monoamines: serotonin, norepinephrine, dopamine
transports GABA and glycine
glutamate transporters (SLC1)
clears out glutamate to prevent excitotoxicity (excess glutamate can damage neurons)
sodium-potassium ATPase (sodium pump)
how energy is provided for transporting neurotransmitters
transport into neurons is an energy-dependent process
energy comes from the sodium pump
includes co-transport of chloride ions and the counter-transport of potassium ions
the ion movement sets the gradient up to allow neurotransmitter reuptake to occur
ATPase
enzyme that breaks down ATP to release energy
pumps sodium ions out and potassium ions in
generates the electrical potential needed for action potentials
presynaptic monoamine transports
presynaptic → different for each one
vesicular → same for all
transporters are sodium-dependent cotransporters
have binding sites for sodium + the monamine
without sodium present, the transporters cannot bind it
importance of monoamine transporters for drugs (normal process)
monoamines have little time to act on receptors before they are recaptured by presynaptic transporters
prevents neurotransmitter overload
importance of monoamine transporters for drugs (drug mechanism)
to increase neurotransmitter activity, drugs block the transporters, so more neurotransmitters stay in the synapse
boosts signaling and therapeutic effects
importance of monoamine transporters for drugs (conditions targeted)
ADHD
unipolar depression
anxiety disorders
pain
erectile dysfunction
OCD
trauma
3 types of intracellular vesicle transporters
vesicular monoamine and acetylcholine transporters
vesicular inhibitory amino acid transporters (VIAATs)
vesicular glutamate transporters (vGluTs 1-3)
vesicular monoamine and acetylcholine transporters
VMATs (monoamines) and VAChT (acetylcholine)
gene family: SLC18
handles serotonin, norepinephrine, dopamine, histamine
vesicular inhibitory amino acid transporters (VIAATs)
gene family: SLC32
handles neurotransmitters like GABA and glycine
vesicular glutamate transporters (vGluTs 1-3)
gene family: SLC17
handles glutamate
vesicular transport
system keeps the charge balance constant, so vesicles remain ready for future neurotransmission
a proton ATPase (proton pump) uses energy to pump H* ions out of the vesicle
this creates a charge gradient (more negative inside)
neurotransmitters use this gradient to enter the vesicle
once inside, neurotransmitters are stored until the next action potential triggers release
why vesicular transporters are drug targets
no known drugs currently target glutamate, ACH, and GABA transporters
vesicular monoamine transporters (VMATs) are important drug targets, increases dopamine signaling, explaining stimulant effects