Quiz #4 - The Lithosphere and Its Pollution
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
GEOSPHERE
all metals, rocks, minerals, soils, landforms, etc., comprising the solid earth from crust to core
inner core: solid Fe-Ni alloy
outer core: liquid Fe-Ni
mantle
crust
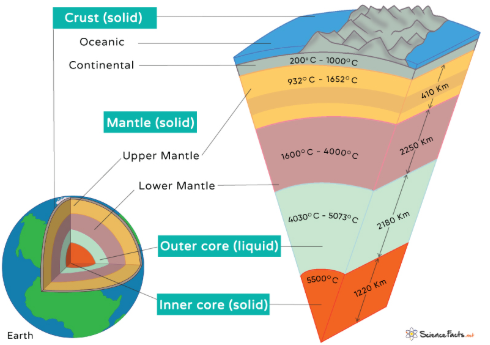
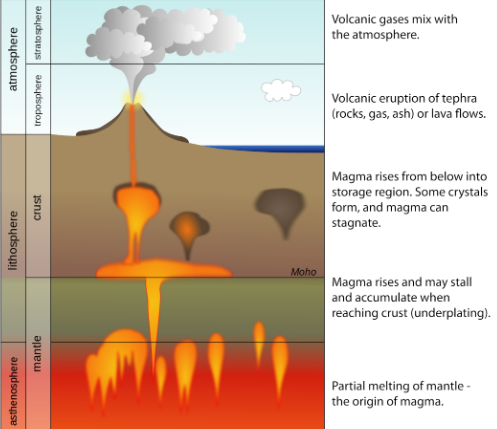
LITHOSPHERE
rigid outer layer of the geosphere
all metals, rocks, minerals, soils, landforms, etc. from the upper mantle to the crust
mostly solid until seismic triggers force magma towards the surface as lava
magma: hot fluid made up of minerals and elements
convection currents and tectonic plate shifts cause magma to push up towards the surface
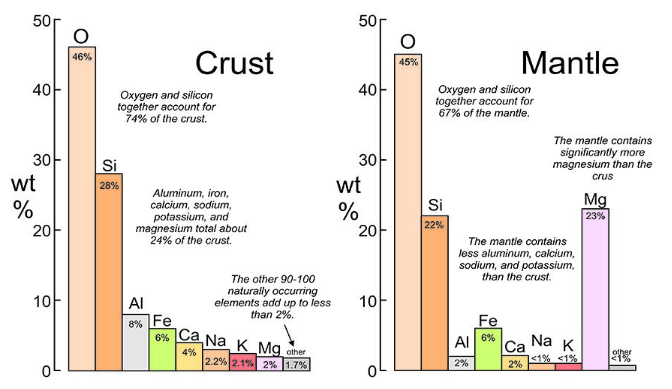
COMPOSITION
oxygen (O) and silicon (Si) are the dominant elements in the lithosphere
mantle
thickest, densest, magnesium (Mg) and iron (Fe) rich
oceanic crust
thinner, denser, magnesium (Mg) and iron (Fe) rich
continental crust
thicker, less dense, aluminum (Al) and silicon (Si) rich
MINERAL GROUPS
naturally-occurring, crystalline, inorganic solids with a specific chemical composition and a highly ordered atomic structure
fundamental building blocks of rocks and are the primary sources of elements used in environmental and industrial processes
minerals are grouped based on the anionic constituent within their chemical formula
ore: naturally-occurring solid material from which a metal or mineral can be profitably extracted
MINERAL GROUPS
silicates: SiO44-
comprise ~90% of Earth’s crust
leads to different structures with varying ratios of silicon (Si) to oxygen (O)
silicates can form a variety of different structures because it coordinates as a tetrahedron
Mining
strip/open-pit mining
Ore Preparation
crushing → washing → sorting
Processing
refined for aluminum production
Use
aluminum cans, aircrafts, ceramics, clays, construction
Concern
land disturbance; high energy use
MINERAL GROUPS
carbonates: CO32-
lime/concrete
calcium carbonate (CaCO3)
calcium magnesium
carbonate CaMg(CO₃)₂
iron ore
iron (II) carbonate (FeCO3)
iron(III) carbonate (Fe₂(CO₃)₃)
Mining
quarry blasting & cutting
Ore Preparation
crushed → sorted by size
Processing
heated for lime/cement; smelt Fe carbonates
Use
construction; soil buffering; steel production
Concern
dust; CO₂ release from heating; weathering
MINERAL GROUPS
oxides: O2–
iron ore
iron (III) oxide (Fe2O3)
iron (II,III) oxide (Fe3O4)
aluminum ore
aluminum (III) oxide (Al2O3)
chromium ore
iron (II) chromite (FeCr₂O₄)
Mining
large-scale open-pit or underground
Ore Preparation
crushing → magnetic/gravity separation
Processing
smelting in furnaces
Use
steel production
Concern
tailings, greenhouse gases
MINERAL GROUPS
sulfides: S2–
cooper ore
copper iron sulfide (CuFeS₂)
lead ore
lead sulfide (PbS)
zinc ore
zinc iron sulfide (Zn,FeS)
nickel ore
iron nickel sulfide ((Fe,Ni)₉S₈)
Mining
underground/open-pit
Ore Preparation
crushing → milling → flotation
Processing
smelting/refining
Use
Cu wiring/pipes, Pb batteries, Zn galvanizing
Concern
acid mine drainage, toxic tailings
MINERAL GROUPS
sulfates: SO42–
hydrous sulfates
calcium sulfate dihydrate (CaSO₄•2H₂O)
anhydrous sulfates
calcium sulfate (CaSO4)
barium sulfate (BaSO4)
Mining
surface quarrying
Ore Preparation
ground to powder
Processing
minimal, as it is often used directly
Use
cement, plaster, drywall, drilling fluid
Concern
dust; habitat disturbance
MINERAL GROUPS
halides: F–, Cl–, Br –, etc.
sodium chloride (NaCl)
calcium fluoride (CaF2)
Mining
room-and-pillar mining; evaporation ponds
Ore Preparation
crushed → washed → sorted
Processing
purified and packaged
Use
table salt, water softeners, remove impurities in steel-making
Concern
sinkholes; brine waste
MINERAL GROUPS
phosphates: PO43-
contains many other minerals; therefore, phosphates have plenty of chemical diversity
ions of similar size and charge can substitute for one another in the crystal structures
calcium phosphate minerals
apatite: Ca5(PO4)3(OH,F,Cl)
Mining
strip mining
Ore Preparation
crushing → washing → flotation
Processing
reaction with an acid
Use
fertilizers, detergents, livestock feed
Concern
eutrophication; radioactive byproducts
MINERAL GROUPS
native elements
metals
platinum (Pt)
iridium (Ir)
osmium (Os)
iron (Fe)
zinc (Zn)
tin (Sn)
gold (Au)
silver (Ag)
copper (Cu)
mercury (Hg)
lead (Pb)
chromium (Cr)
platinum (Pt)
iridium (Ir)
osmium (Os)
gold (Au)
siderophiles
elements that sink to the core to dissolve in iron (Fe)
semimetals
bismuth (Bi)
antimony (Sb)
arsenic (As)
tellurium (Te)
selenium (Se)
nonmetals
sulfur (S)
carbon (C) (diamond, graphite, amorphous)
Mining
placer or hard-rock mining
Ore Preparation
gravity sorting → crushing → milling
Processing
smelting, refining, cutting/polishing
Use
jewelry, electronics, lubricants, etc.
Concern
cyanide (CN-) or mercury (Hg) contamination, sediment disruption, water demand
IMPURITIES IN MINERALS
color variations in minerals is due to impurities within the chemical composition
“Impurities such as silicon dioxide (SiO2), iron oxides (FexOy), and graphite (C) give marble its color and characteristic rich veining and clouding.” – Britannica
Rutilated quartz, is made of silicon dioxide (SiO2), with titanium dioxide (TiO2).
The trace presence of calcium (Ca), iron (Fe), zinc (Zn), chromium (Cr), magnesium (Mg), etc. give Himalayan salt its pink, reddish, or beet-red color.
“All natural diamonds contain some nitrogen (N) impurities. The traditional classification of natural diamonds is based on their nitrogen (N) content.” – Chemical Review, 2020
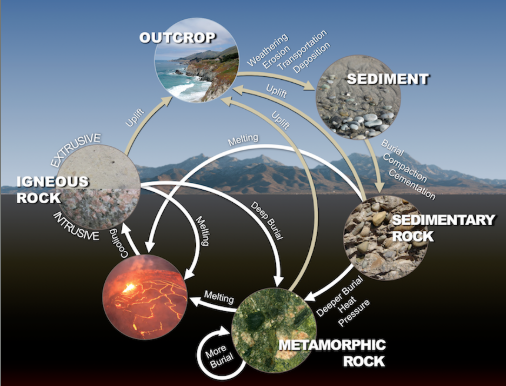
ROCKS
naturally-occurring aggregate of different minerals that fused together due to high pressures and temperatures over a long period of time
rocks are grouped by how they were formed
Igneous
magma (molten rock) that has either cooled slowly underground or quickly at the surface
Sedimentary
the weathered products of other rocks accumulating at the surface and then buried by other sediments
Metamorphic
igneous or sedimentary rocks that form new minerals (therefore new rocks) with heat and pressure
THE ROCK CYCLE IN ACTION
examples:
limestone is a sedimentary rock
calcium carbonate (CaCO3) fossils
shells, sand, and mud deposited at the bottom of oceans and lakes solidify into rock
marble is a metamorphic rock
sedimentary limestone (CaCO3) undergoes pressure and heat so that the grains recrystallize
coloration is due to impurities within the chemical composition
ORGANIC MATTER
percentage of soil that consists of decomposing plant and/or animal tissue
primarily composed of carbon (C), hydrogen (H), and oxygen (O) with humus being the final product
humus: stable organic matter that is resistant to further degradation
role in soil structure, water retention, and nutrients in the form of nitrogen (N)
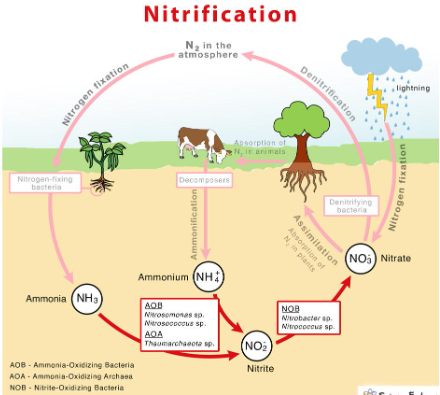
NITROGEN CYCLE
transformations of nitrogen (N) moving through the spheres
atmosphere, lithosphere, hydrosphere, and biosphere
primarily driven by specialized bacteria
nitrogen fixation converts atmospheric nitrogen (dinitrogen: N₂) into bioavailable forms
triple bonds are shorter and stronger than double bonds
nitrogen (N2) does not react readily because it is a strongly bonded, stable compound
REMEMBER THIS IMAGE! Nitrogen cycle = creation of N2
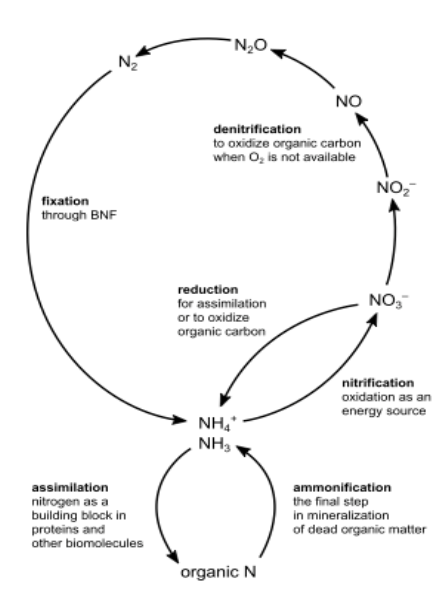
NITROGEN CYCLE: NITROGEN FIXATION
atmospheric dinitrogen (N2) converts to ammonia (NH3) / ammonium (NH4+)
N2 🡪 NH3 / NH4+
enzyme-catalyzed reduction by microorganisms
microbes with the enzyme nitrogenase can use electrons and protons from the microbial metabolism to convert dinitrogen (N2) to ammonia (NH3)
NITROGEN CYCLE: NITRIFICATION
ammonia (NH3) / ammonium (NH4+) to nitrite (NO2-) and then nitrate (NO3-)
NH3 / NH4+ 🡪 NO2- 🡪 NO3-
enzyme-catalyzed oxidation by microorganisms
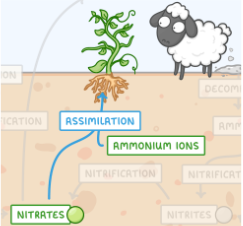
NITROGEN CYCLE: ASSIMILATION
ammonia (NH3) / ammonium (NH4+) and nitrate (NO3-) yielding organic nitrogen compounds (R-NH2)
NH3 / NH4+ / NO3- 🡪 R-NH2
assimilated by bacteria, fungi, algae, and plants
Amino acids: these organic nitrogen (N) compounds found in the environment can move up the food chain to higher organisms
NITROGEN CYCLE: AMMONIFICATION
conversion of organic nitrogen compounds (R-NH2) into ammonia (NH3) /ammonium (NH4+)
R-NH2 🡪 NH3 / NH4+
decomposed by fungi and microorganisms
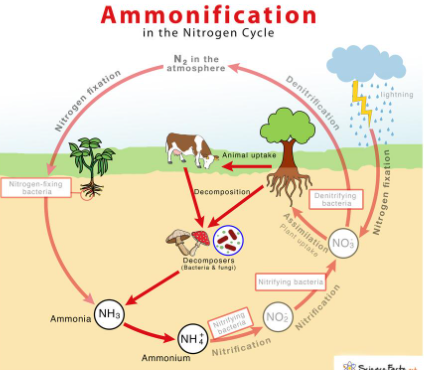
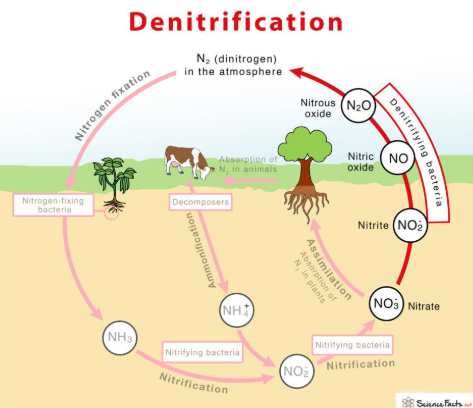
NITROGEN CYCLE: DENITRIFICATION
removing bioavailable nitrogen (N) and returning it to the atmosphere
NO3- 🡪 NO2- 🡪 NO 🡪 N2O 🡪 N2
anaerobic reduction by microorganisms
Denitrifying bacteria:
nitrate (NO3-)
nitrite (NO2-)
nitric oxide (NO•)
nitrous oxide (N2O)
dinitrogen (N2)
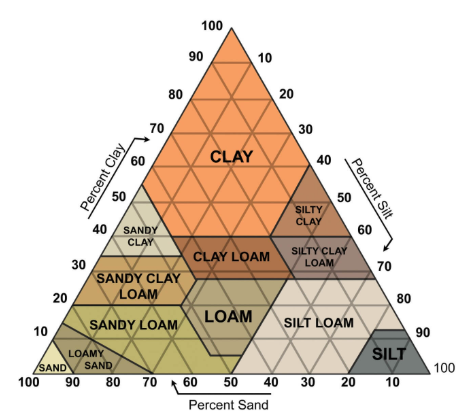
SOIL TEXTURE
determine soil class based on the percentage of clay, silt, and sand
primarily composed of silicates (SiO2)
water retention
porosity
nutrient
resource use
CLAY |
|
SILT |
|
SAND |
|
fine coarse |
|
FOR EXAMPLE:
100% SOIL = 35% CLAY + 25 % SAND + 40% SILT
SOIL: COMPOSITION
rock particles: 45-50%; contains minerals; mostly silicates
air: oxygen (O2) for plants and animals; water storage spaces
water: sustains plant and animal life
organic matter: nutrients
SOIL: FACTORS
climate: temperature, rainfall, etc.
organisms: flora and fauna
relief: topography and slope stability
parent material: type of bedrock
time: how long in place
SOIL: SOIL CHEMISTRY
Nitrogen Cycle
Soil Texture Triangle
Cation Exchange Capacity
CATION EXCHANGE CAPACITY
capacity of the soil to hold on to cations
held by negatively charged clay and organic matter particles in the soil via electrostatic forces
cations are easily exchangeable with other cations
(drier climate = higher salinity)
calcium (Ca2+) -
magnesium (Mg2+)
potassium (K+)
🡪 nutrient cations plants use in the largest amounts
sodium (Na+)
🡪 higher [Na+] in drier climates
iron (Fe2+)
manganese (Mn2+)
zinc (Zn2+)
copper (Cu2+)
🡪 micronutrients for plants
ammonium (NH4+)
🡪 low [NH4+] due to nitrification
hydrogen (H+)
aluminum (Al3+)
🡪 detrimental effects
![<ul><li><p><span style="background-color: transparent;">capacity of the soil to hold on to cations</span></p></li><li><p><span style="background-color: transparent;">held by negatively charged clay and organic matter particles in the soil via electrostatic forces</span></p></li><li><p><span style="background-color: transparent;">cations are easily exchangeable with other cations</span></p></li><li><p><span style="background-color: transparent;">(drier climate = higher salinity)</span><br></p></li></ul><ul><li><p><span style="background-color: transparent;">calcium (Ca<sup>2+</sup>) - </span></p></li><li><p><span style="background-color: transparent;">magnesium (Mg<sup>2+</sup>)</span></p></li><li><p><span style="background-color: transparent;">potassium (K<sup>+</sup>)</span></p></li><li><p><span style="background-color: transparent;">🡪 nutrient cations plants use in the largest amounts</span></p></li></ul><p></p><ul><li><p><span style="background-color: transparent;">sodium (Na<sup>+</sup>)</span></p></li><li><p><span style="background-color: transparent;">🡪 higher [Na<sup>+</sup>] in drier climates</span><br></p></li></ul><ul><li><p><span style="background-color: transparent;">iron (Fe<sup>2+</sup>)</span></p></li><li><p><span style="background-color: transparent;">manganese (Mn<sup>2+</sup>)</span></p></li><li><p><span style="background-color: transparent;">zinc (Zn<sup>2+</sup>)</span></p></li><li><p><span style="background-color: transparent;">copper (Cu<sup>2+</sup>)</span></p></li><li><p><span style="background-color: transparent;">🡪 micronutrients for plants</span></p></li></ul><p></p><ul><li><p><span style="background-color: transparent;">ammonium (NH<sub>4</sub><sup>+</sup>) </span></p></li><li><p><span style="background-color: transparent;">🡪 low [NH<sup>4+</sup>] due to nitrification</span></p></li></ul><p></p><ul><li><p><span style="background-color: transparent;">hydrogen (H<sup>+</sup>)</span></p></li><li><p><span style="background-color: transparent;">aluminum (Al<sup>3+</sup>)</span></p></li><li><p><span style="background-color: transparent;">🡪 detrimental effects</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/bd1a6b1e-72aa-4d58-ac36-bf84eea99304.png)
LITHOSPHERIC POLLUTION
ACID DEPOSITION
acidifying soils
erosion, sinkholes, caves, and underground rivers
leaching toxic metals and nutrients
MINING & EXTRACTING
disturbance of soils and bedrock and habitats
generation of tailings and mine waste
acid mine drainage
hydraulic fracturing
DOMESTIC WASTE
solid municipal waste in landfills
AGRICULTURE
fertilizers causing nutrient pollution
pesticides, herbicides, etc. causing chemical pollution
DRY & WET ACID DEPOSITION
DRY DEPOSITION
acidic gas, dust, or particles, typically from SOx (g) and NOx (g) species, that deposit directly onto surfaces
form acids in situ when in contact with surface moisture (dew, water films, wet leaves, etc.)
WET DEPOSITION
acidic substances dissolved in precipitation (rain, snow, sleet, fog, or hail) that fall to Earth
gases SOx (g), NOx (g), and CO₂ (g) dissolve in atmospheric water droplets and react to form acids
SULFUR OXIDES (SOx)
SO2 (g) 🡪 SO3 (g) 🡪 H2SO4 (aq)
NITROGEN OXIDES (NOx)
2 NO• (g) 🡪 2 NO2 (g) 🡪 HNO3 (aq)
CARBON DIOXIDE
CO2 (g) 🡨🡪 H2CO3 (aq)
SULFATE (SO42-) DEPOSITION IN THE U.S.
using National Atmospheric Deposition Program (NADP) and Clean Air Status and Trends Network (CASTNET)
reduced sulfate (SO42-) deposition is a result of the Clean Air Act (1970) and its amendments (1990)
NITRATE (NO3-) DEPOSITION IN THE U.S.
using National Atmospheric Deposition Program (NADP) and Clean Air Status and Trends Network (CASTNET)
“reduced” nitrate (NO3-) deposition is a result of the Clean Air Act (1970) and its amendments (1990)
LITHOSPHERIC DISSOLUTION
calcium sulfate: CaSO4
calcium nitrate: Ca(NO3)2
calcium bicarbonate: Ca(HCO3)2
Calcium carbonate (CaCO3) reacts with acid deposition (H2SO4, HNO3, or H2CO3) causing chemical weathering.
Calcium bicarbonate (Ca(HCO₃)₂) is moderately soluble, releasing calcium (Ca²⁺) and bicarbonate (HCO₃⁻) ions into soils and waterways where they enhance carbonate weathering and influence the carbon cycle.
CaCO3 (s) + H2SO4 (aq) 🡪 CaSO4 (s) + H2O(l) + CO2 (g)
CaCO3 (s) + 2 HNO3 (aq) 🡪 Ca(NO3)2 (aq) + H2O(l) + CO2 (g)
CaCO3 (s) + H2CO3 (aq) 🡪 Ca(HCO3)2 (aq)
SINKHOLES, CAVES, & UNDERGROUND RIVERS
“Map shows karst areas of the continental United States having sinkholes in soluble rocks (carbonates and evaporites), as well as insoluble volcanic rocks that contain sinkholes. The volcanic bedrock areas contain lava tubes that are voids left behind by the subsurface flow of lava, rather than from the dissolution of the bedrock.” – USGS 2020
carbonate (CO32-)
evaporite: sulfates (SO42-) and halides (Cl-)
volcanic: silicates (SiO44-)
(Sinkholes are showing that soluble carbonate rocks are dissolving due to dry and wet acids.)
LITHOSPHERIC LEACHING
NUTRIENT LEACHING
sulfate (SO42-), nitrate (NO3-), and carbonate (CO32-) can attach to cations in soil making these cations unavailable, thus potentially removing nutrients
hydrogen ion (H+) can then take the place of these cations, acidifying the soil
TOXIC CATION LEACHING
hydrogen ion (H+) can displace toxic metal cations, acidifying the soil
toxic metal cations are even more soluble and mobile in acidic environments and will continue leaching into biological systems
The lithosphere is mostly made of silicates.
Plant roots actively use an acid-driven process called cation exchange to acquire essential nutrients. However, an excess of certain acidic cations, like aluminum, can become toxic and cause plant death, especially in highly acidic soils.
TOXIC METALS, LEACHING, & ACIDIFICATION
soil acidification
nutrient leaching
toxic metal leaching
loss of habitats and/or food sources, limiting biodiversity and causing food chain imbalances
Critical pHs
Frogs can survive at pHs ~4
However, their food source (mayflies) will not survive at pH ≤ 5.5
ACID MINE DRAINAGE
occurs primarily when sulfide (S2-) minerals in mine tailings, waste rock, or exposed bedrock are oxidized by water (H2O) and oxygen (O2)
release hydrogen ions (H+) which lower the pH (~2.5 – 4) and maintain the solubility of the ferric ion (Fe3+)
dissolves heavy metals like copper (Cu) and mercury (Hg) into the groundwater and/or surface water
if the pH were to increase, ferric ion (Fe3+) precipitates as iron(III) hydroxide (Fe(OH)3)
DON’T have to know these reactions:
2 FeS2 (s)(+ 7 O2 (g) + 2 H2O(l) 🡪 2 Fe2+(aq) + SO42-(aq) + H+(aq)
4 Fe2+(aq) + O2 (g) + 4 H+(aq) 🡪 4 Fe3+(aq) + 2 H2O (l)
FeS2 (s) + 14 Fe3+(aq) + 8 H2O (l) 🡪 15 Fe2+(aq) + 2 SO42-(aq) + 16 H+(aq)
FeS2: iron disulfide (pyrite)
Fe2+: ferrous ion
Fe3+ : ferric ion
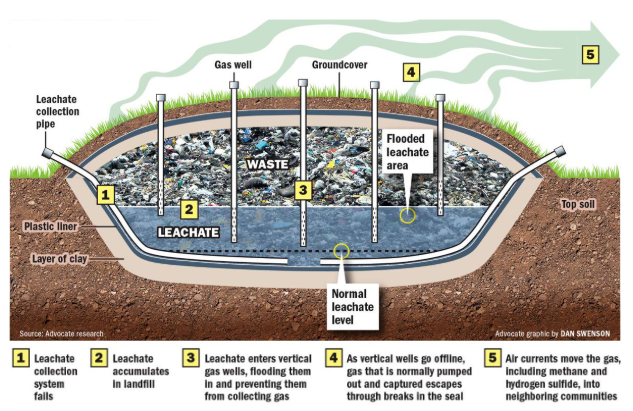
LANDFILLS
landfill: large hole in the ground that holds solid waste and is covered by soil/clay
leachate: water that leaches into a landfill and liquid that drains from a landfill
methane (CH4) and carbon dioxide (CO2) are primary gases produced from the decomposition of organic matter
2 H2S + 3 O2 → 2 SO2 + 2 H2O
WET/DRY ACID DEPOSITION
SO2 → SO3 → H2SO4
COMPOSITION OF REFUSE
refuse: solid waste (typically nonhazardous) that is collected and transported to a disposal site
cellulose: a long chain of repeating glucose (sugar) molecules
hemicellulose: made up of a variety of repeating sugars
lignin: carbon-rich polymer that resists decay; main component of humus which is the final product of organic matter decomposition
methane (CH4) and carbon dioxide (CO2) are primary gases produced from the decomposition of organic matter
FOUR STAGES OF LANDFILL DECOMPOSITION: AEROBIC PHASE
Four stages of decomp NOT on upcoming quiz!!!
depletion of oxygen (O2)
temperature increase
high carbon dioxide (CO2) production
minimal loss of solids
pH is ~5.5 - 6.5
(CH2O)N + H2O → (CH2O)n + (CH2O)n
(CH2O)n is the empirical formula for most sugars
Where… “N” represents a long polysaccharide chain + “n” represents a smaller chain than “N”
(CH2O)n + n O2 → n CO2 + n H2O + heat
Where… “n” represents a smaller chain than “N” for the long polysaccharide chain shown in previous slides
FOUR STAGES OF LANDFILL DECOMPOSITION: ANAEROBIC ACID PHASE
Four stages of decomp NOT on upcoming quiz!!!
no oxygen (O2) infiltration
organic acids accumulate
minimal carbon dioxide (CO2) production
possible hydrogen (H2) production
minimal loss of solids
pH is ~5.0 – 6.0
fermentation: microorganisms breaking down sugars to acetic, lactic, and formic acids (-COOH), alcohols (-OH), and gases (CO2 and H2)
products are dependent on many variables
FOUR STAGES OF LANDFILL DECOMPOSITION: ACCELERATED METHANE PHASE
Four stages of decomp NOT on upcoming quiz!!!
high methane (CH4) production
gas composition is 50:50 methane (CH4)/carbon dioxide (CO2)
pH is ~7 - 8
significant solid decomposition begins
microorganisms decompose organic acids (-COOH) to methane (CH4) and carbon dioxide (CO2)
2 CH3COOH 🡪 CH4 + 3 CO2
acetic acid
2 C3H6O3 🡪 3 CH4 + 3 CO2
lactic acid
HCOOH 🡪 H2 + CO2
formic acid
FOUR STAGES OF LANDFILL DECOMPOSITION: DECELERATED METHANE PHASE
Four stages of decomp NOT on upcoming quiz!!!
decrease in methane (CH4) production
pH is > 7
significant solid decomposition but slower rate
STABILIZATION
degradable solids consumed
oxygen (O2) infiltrates
geological timescale
CRUDE OIL & NATURAL GAS WELLS
drilling: extraction of natural gas and/or crude oil (fossil fuels) from the lithosphere by drilling a hole to reach a reservoir, and then using tubing to pump the oil or gas to the surface
conventional drilling: vertical well
unconventional drilling: horizontal wells
hydraulic fracturing (“fracking”): “crack open rocks deep below the earth’s surface to access trapped fossil fuel deposits” - Natural Resources Defense Council (NRDC)
FRACKING FLUID
freshwater with proppants, base fluid, and additives
proppants: allows oil or gas to flow
sand
ceramic pellets
small incompressible particles
base fluid: “applies pressure … to the fractures”
water
oil
methanol
liquid carbon dioxide
liquified petroleum gas
additives: “maintain the integrity of the oil or gas” and equipment
biocides: inhibit microorganism growth
acids: dissolve minerals and debris
pH adjustors
SEISMIC ACTIVITY
high pressure injection of wastewater (and hydraulic fracturing fluid) and into naturally-occurring faults, as well as natural or man-made fractures
Fractures can’t be controlled!
Fractures can be upwards of…
~ 6 mm in width
~ 400 m in length
~100 m in height
1 meter = 3.28 ft
WATER USAGE & CONTAMINATION
ground and surface water sources are drained
large volume of wastewater is produced
fractures cause groundwater and surface water contamination
gas, oil, hydraulic fracturing fluid, and radionuclides
radionuclides: “an unstable atom that releases high energy radiation as it breaks down to become more stable” – National Cancer Institute
naturally-occurring radioactive materials (NORMs), such as uranium (U), thorium (Th), and radium (Ra), that are found in gas and oil deposits and can dissolve in water (H2O)
SOIL PURIFICATION: BURIAL
IN SITU: removal of the contaminant(s) while soil is in its original place
EX SITU: removal of contaminated soil prior to removing the contaminant(s)
Burial & Capping
migration of pollutants can occur by:
rainwater moving through the soil
surface water moving over the ground
wind blowing across the site
covers the area with concrete, asphalt, clay or a geomembrane material preventing the migration of pollutants from the contamination site
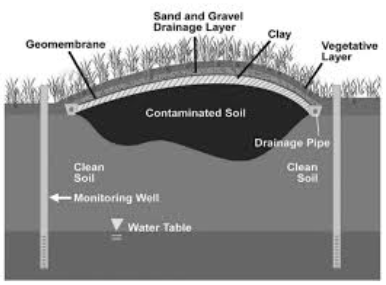
SOIL PURIFICATION: IMMOBILIZE
Solidification & Vitrification
“… adds a binder to the media … change the physical properties of the media … results in a decrease in its permeability and an increase in its compressive strength."
Federal Remediation Technologies Roundtable
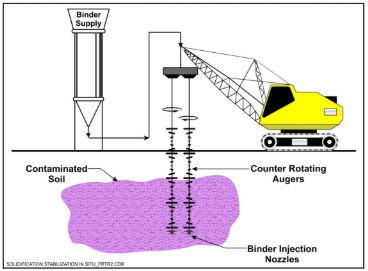
SOIL PURIFICATION: MOBILIZE
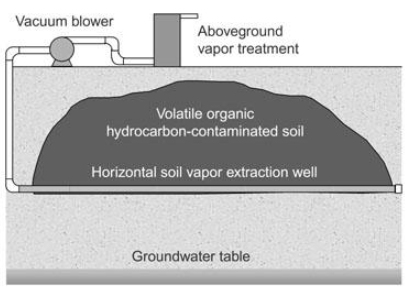
SOIL PURIFICATION: DESTROY
Incineration & Bioremediation
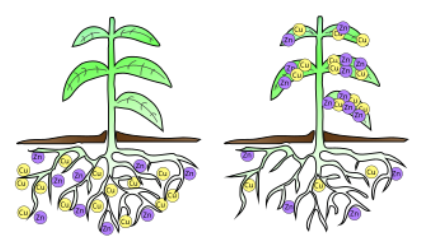
Phytoremediation = uses plants to remove or contain contaminants
CONTAMINANTS
metals
pesticides
solvents
explosives
crude oil and its derivatives
PLANTS
mustard plants
alpine pennycress
hemp
pigweed