Energy Changes
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Exothermic
energy is transferred to surroundings
What are examples of an exothermic reaction?
combustion reactions
many oxidation reactions
most neutralisation reactions
respiration
Combustion
the process of burning by heat
oxidation
the gain of oxygen, or loss of electrons, by a substance during a chemical reaction.
neutralisation reactions
The reaction between an acid and a base to form a salt plus water.
Endothermic
energy is taken in from surroundings
Examples of an endothermic reaction
thermal decomposition reactions
photosynthesis
instant ice packs
thermal decomposition
a compound breaks down to form two or more substances when it is heated
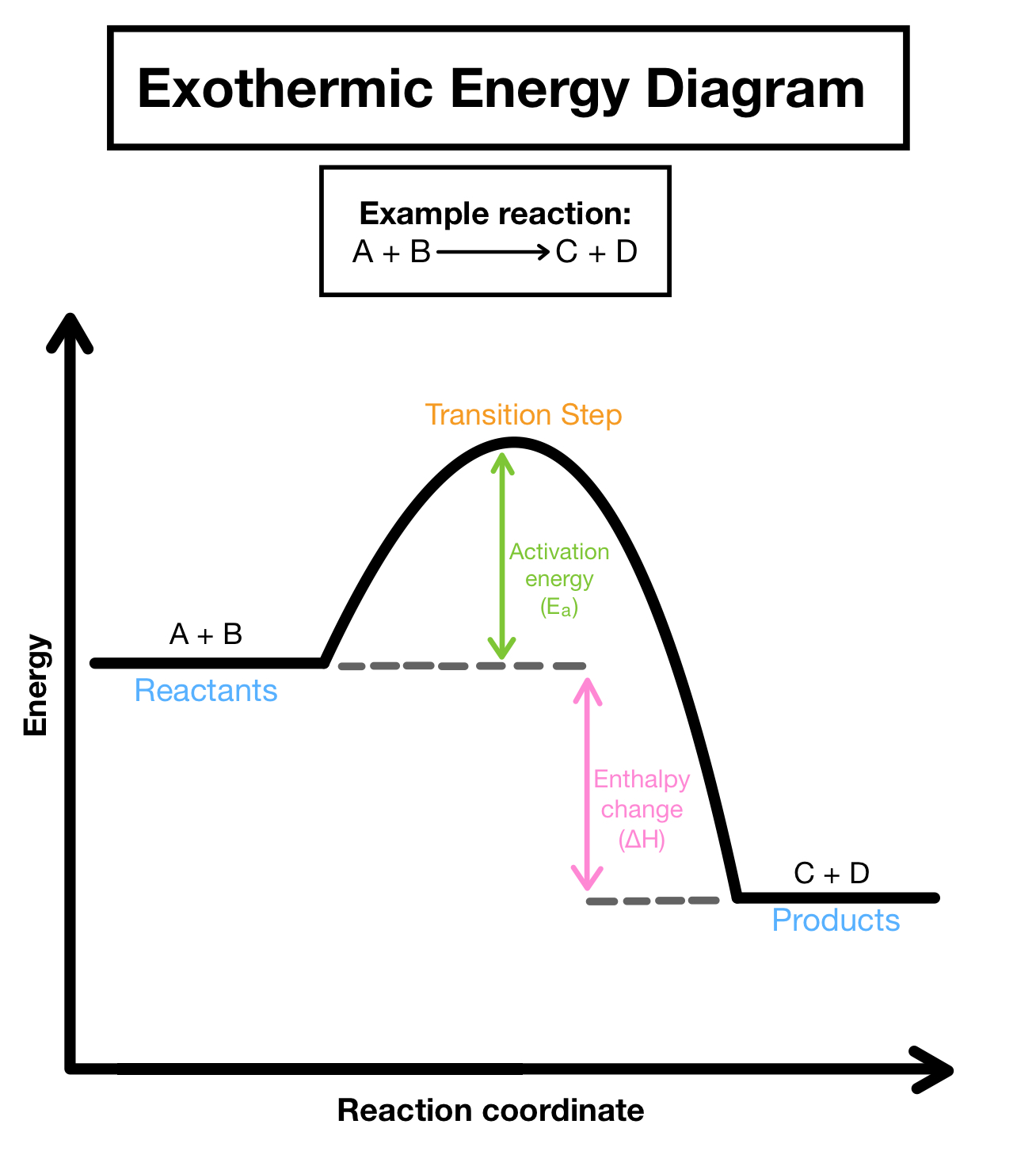
exothermic reaction profile
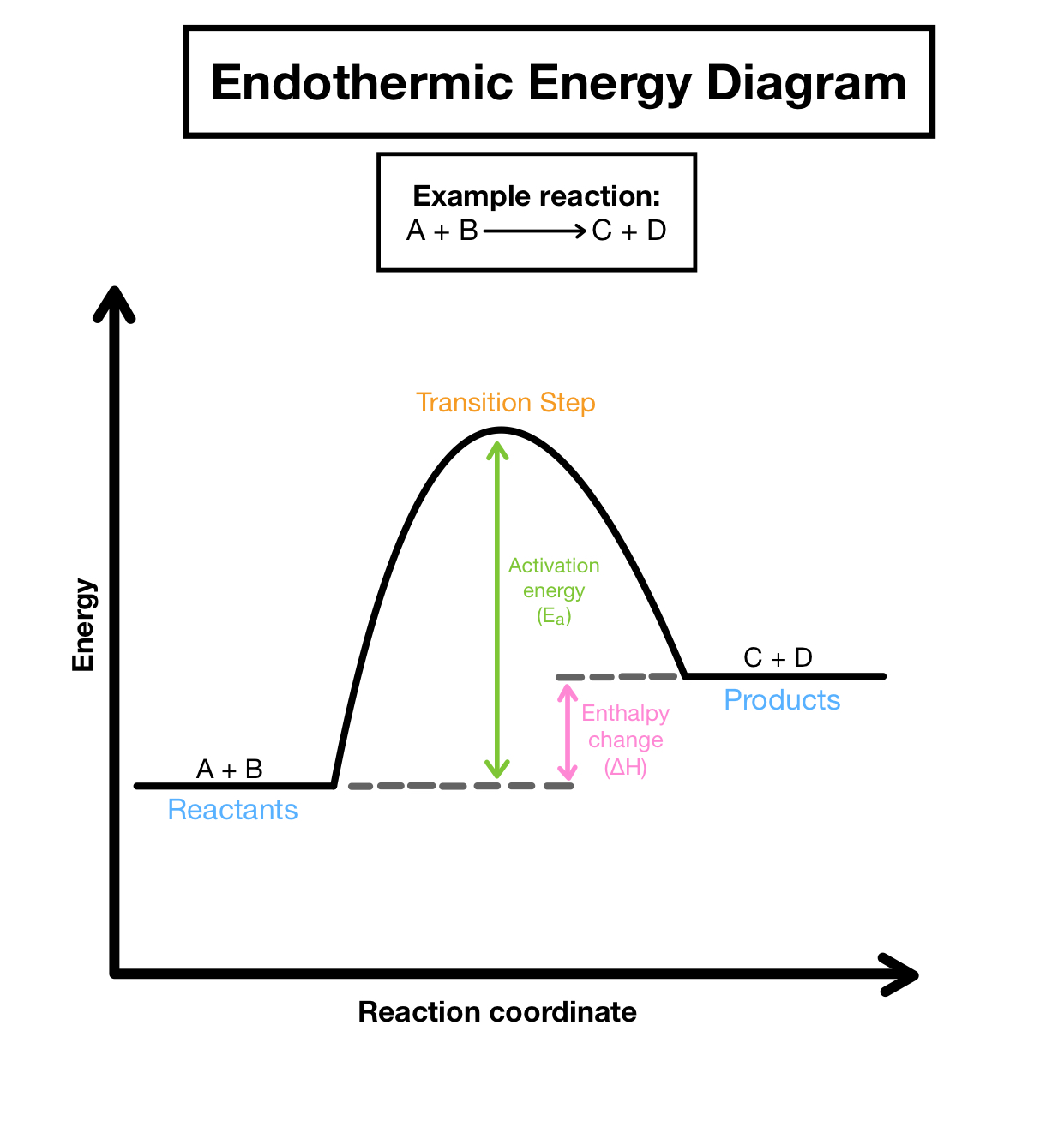
endothermic reaction profile
activation energy
The minimum amount of energy that colliding particles must have for them to react.
What type of reaction breaks bonds?
endothermic
What type of reaction forms bonds?
exothermic
How do you calculate energy change?
add together the bond energies for all the bonds in the reactants - this is the 'energy in'
add together the bond energies for all the bonds in the products - this is the 'energy out'
energy change = energy in - energy out