19 Cancer Chemotherapy Part 1 - Cytotoxic Agents
1/131
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
132 Terms
General targets for cancer chemotherapy
-rapidly dividing cells
-most sensitive tissues/cells
Treatment modalities available for cancer
-surgery
-radiation
-chemotherapy
-biologic therapy
-also combo therapy of the above options
why do we often use combination therapy to treat cancer?
-minimize toxicities
-prevent resistance
-take advantage of different MOAs
goals of cancer therapy based on stage of cancer
Phase 0, 1, 2, and early 3 (localized regional disease):
-cure
-control tumor growth and spread
-inhibit disease recurrence
Late phase 3 or phase 4 (advanced or metastasized):
-palliate sxs
-reduce tumor load
-prolong survivial
-increase quality of life
what is unique about treatment in stage 0 cancer?
often not treated, but rather is monitored until clinically apparent
What is the general reason for the classic AEs of cancer chemotherapy?
AEs manifest due to death of highly proliferative cells (hair follicles, GI lining, blood cells) b/c chemo agents are designed to kill rapidly dividing cells
Classic AEs of chemotherapy
-alopecia
-N/V/D
-myelosuppression
-infertility
-stomatits
-neurotoxicity
-nephrotoxicity
-hepatotoxic
-pulm. toxicitiy
difference b/w cell cycle specific and cell cycle nonspecific cytotoxic agents
cell cycle specific:
-most active against cells in a specific phase
cell cycle nonspecific:
-most effective against actively dividing cells
-also effective in G0 phase
different MOAs of cytotoxic agents (not any specific drugs, just the potential MOAs that cytotoxic agents can exhibit)
-block DNA synthesis
-DNA damage
-inhibit microtubule function
-inhibit topoisomerase function
major types of cytotoxic agents
-alkylating agents
-antitumor biologics
-antheracyclines
-topoisomerase 1 inhibitors
-antitumor ABX
-antimetabolites
-taxanes
-vinca alkaloids
-topo 2 inhibitors
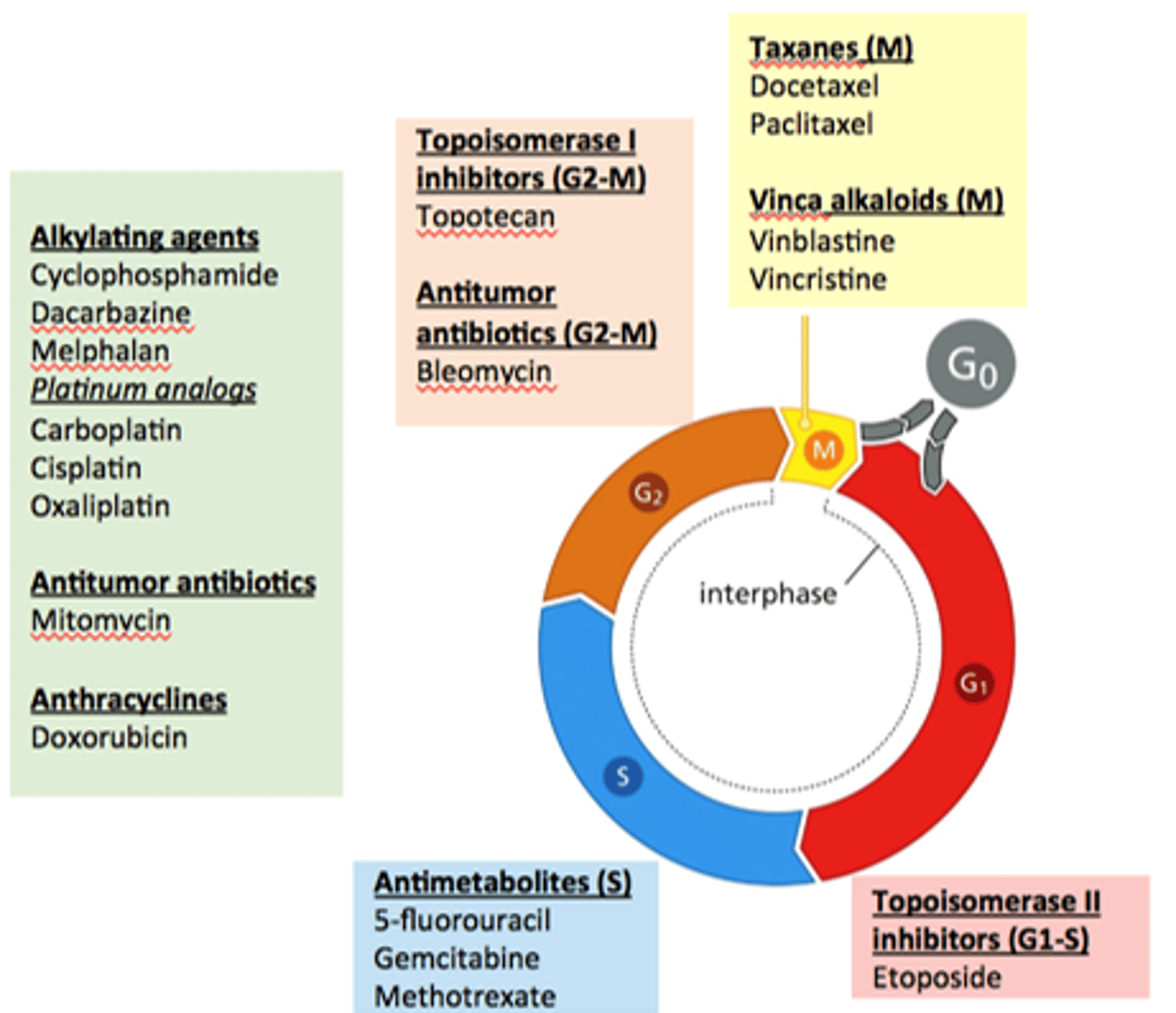
which agents are alkylating agents?
-cyclophosphamide
-dacarbazine
-melphalan
-platinum analogues
-carboplatin
-cisplatin
-oxaplatin
which agents are antitumor biologics?
-mitomycin, bleomycin
which agents are anthracyclines?
doxarubicin
which agents are topo 1 inhibitors and which phase(s) do they work in?
Tocotecan, irinotecan (G2-M)
which agents are antitumor ABX and what phase(s) of the cell cycle do they work in?
bleomycin (G2-M)
which agents are antimetabolites for cancer?
-5 fluorouracil
-gemcitabine
-methotrexate
which agents are taxanes and which phase(s) of the cell cycle do they work in?
-docetaxel
-paclitaxel
work in M phase
which agents are topo 2 inhibitors for cancer and which phase(s) of the cell cycle do they work in?
etoposide (G1-S)
Are DNA alkylating agents cell cycle specific or nonspecific?
Nonspecific
MOA of DNA alkylating agents for cancer
-inhibit DNA synthesis by covalently crosslinking DNA or RNA strands
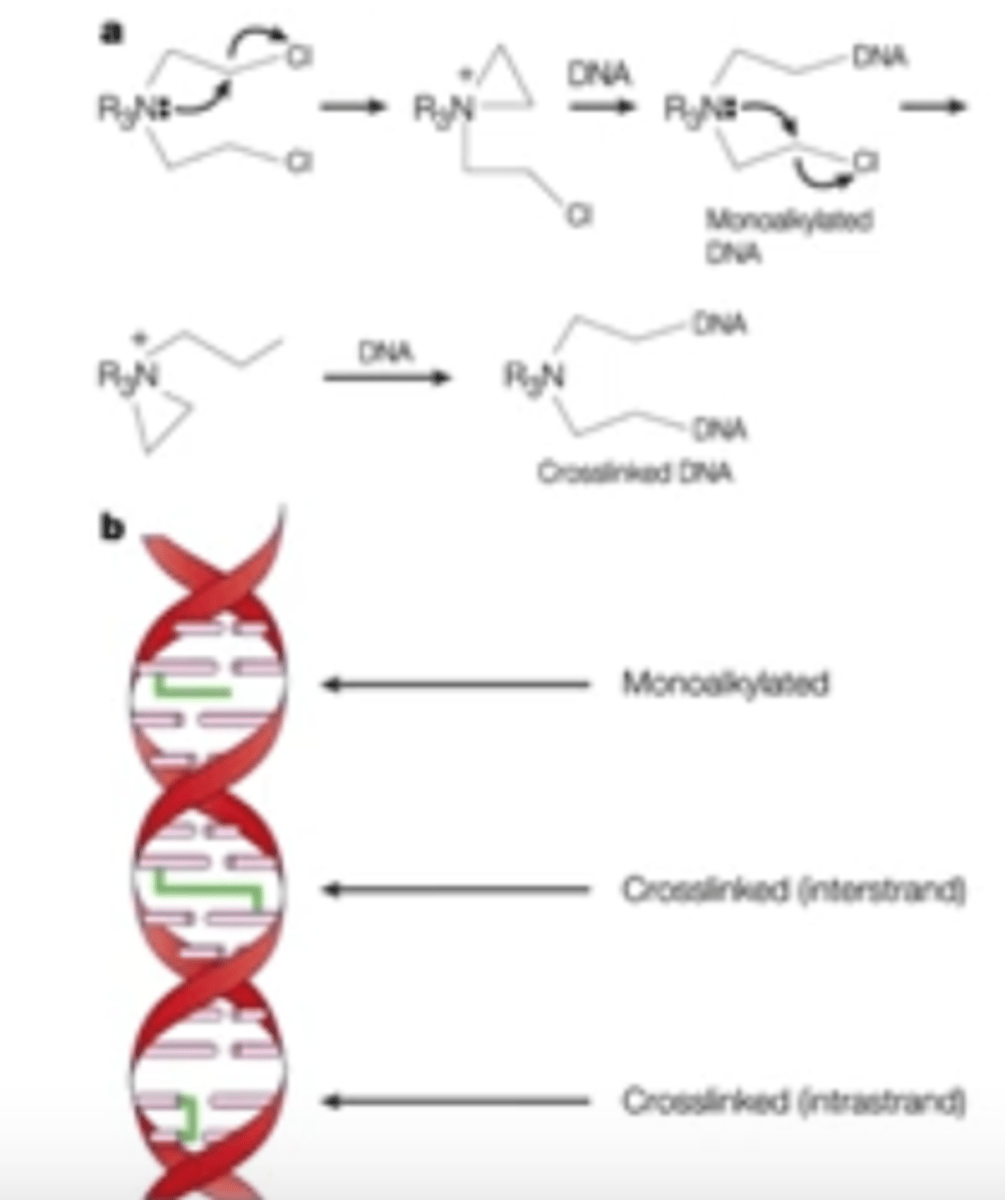
Which specific cancers do DNA alkylating agents treat?
Not specific; treat a wide variety
Major class toxicities of DNA alkylating agents
-bone marrow tox
-GI tox
-N/V
which DNA alkylating agents are "classic"? (consider drug class and specific drugs)
classic:
-Nitrogen mustards (cyclophosphamide; ifosfamide)
-Nitrosoureas (carmustine)
which DNA alkylating agents are nonclassic?
-dacarbazine
-temozolamide
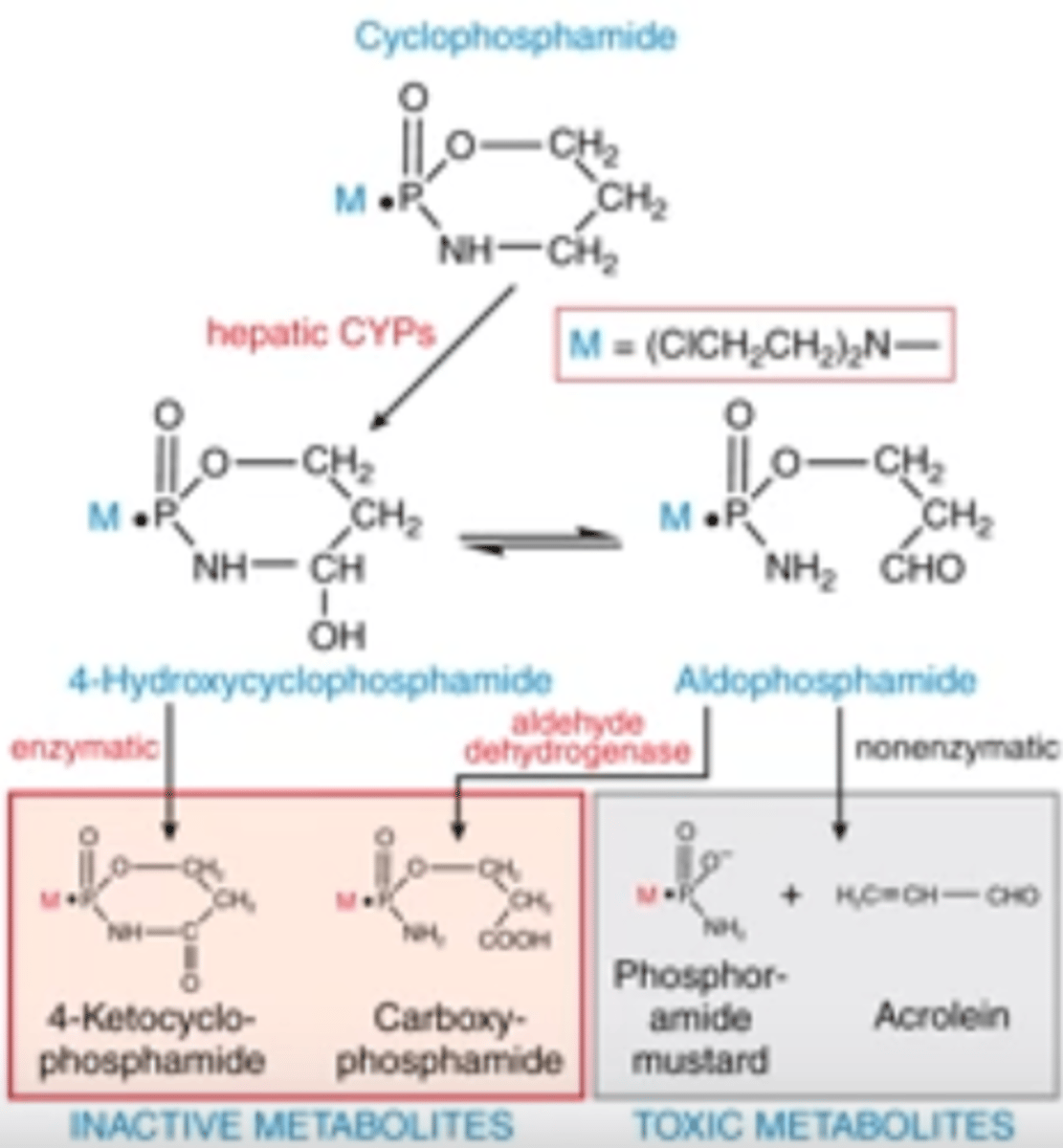
MOA of cyclophosphamide
PRODRUG that makes cytotoxic metabolites
classic (nitrogen mustard) DNA alkylating agent that crosslinks DNA and inhibits DNA synthesis
what are the cytotoxic metabolites made by cyclophosphamide
-phosporamide mustard
-acrolein
pharmacophore of cyclophosphamide
nitrogen mustard group
ROA of cyclophosphamide
PO
IV
AEs of cyclophosphamide
-myelosuppression
-N/V
-hemorrhagic cysts due to acrolein toxicity
spectrum of cyclophosphamide activity
-ALL
-CLL
-NHL
-HL
-myeloma
-myeloblastoma
-testicular
-breast
-ovary
-lung
-cervix
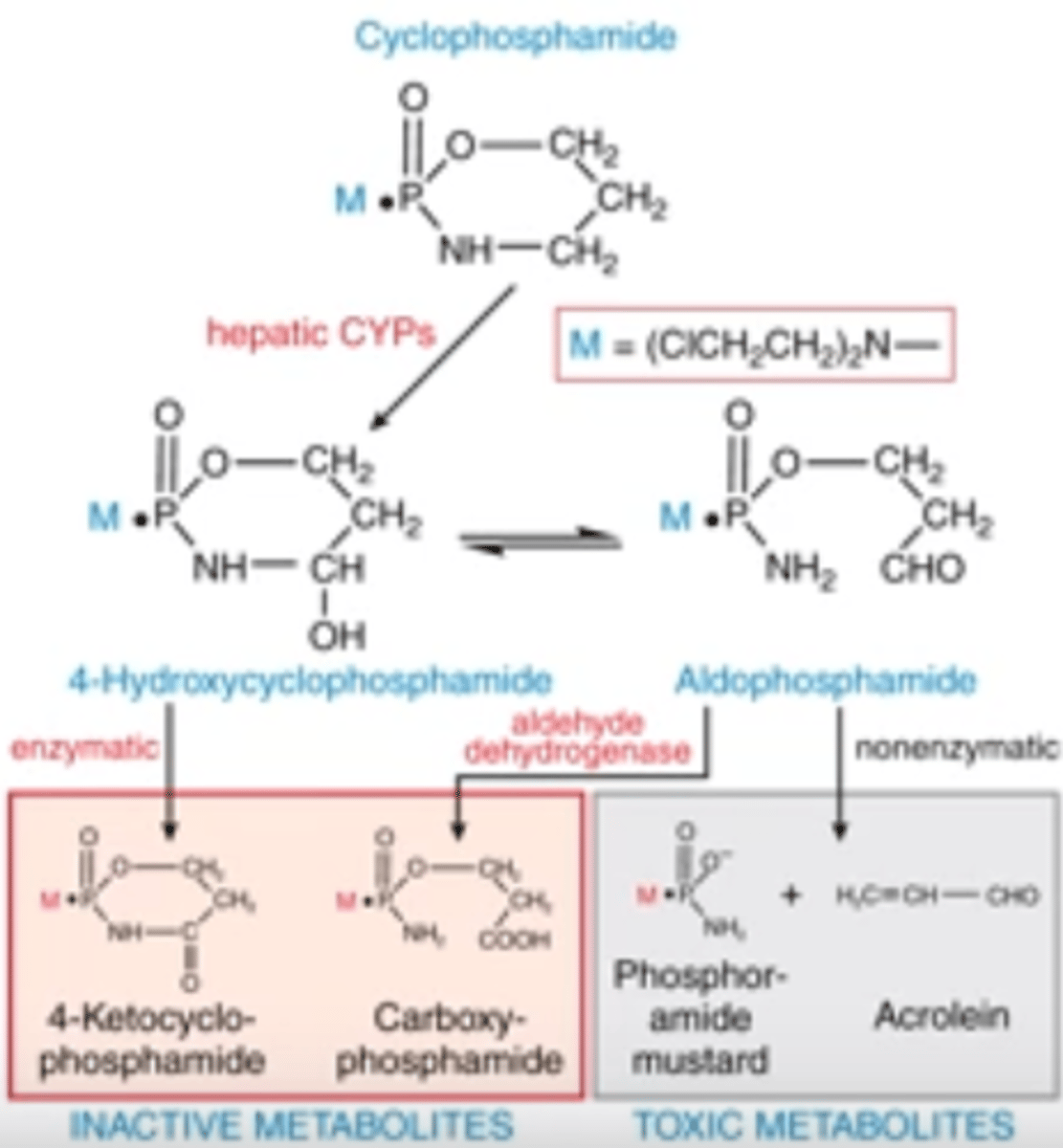
How does cyclophosphamide become activated?
What are the metabolites?
-describe the entire pathway beginning from prodrug to toxic metabolites
-enzymatic and non-enzymatic activity produce active and inactive metabolites
-toxic metabolites are those that confer the cytotoxic activity; hepatic CYPs perform 1st rxn, then nonenzymatic rxn produces toxic metabolites from aldophosphamide
-toxic metabolites are phosphoramide mustard and acrolein
which agents are nitrosoureas?
-carmustine
-lomustine
-streptozocin
which nitrosourea is naturally-occurring, and what makes it unique?
streptozocin is a naturally-occurring nitrosourea with minimal bone marrow toxicity
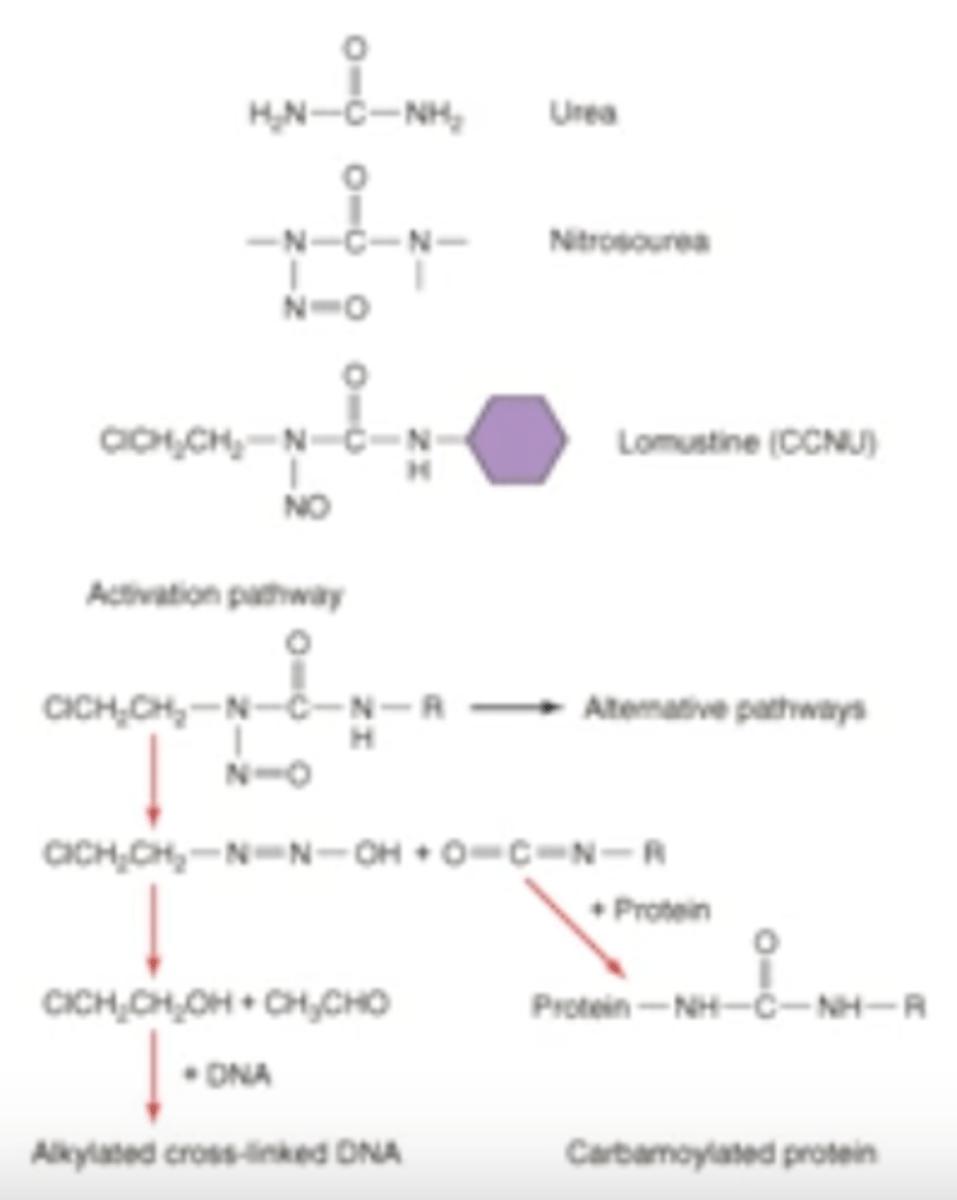
how do nitrosoureas work?
all require activation in vivo into metabolites w/ both alkylating and carbamoylating activities:
-alkylate DNA
-carbamoylate proteins
MOA of nitrosoureas (photo for reference)
are nitrosoureas lipophilic or hydrophilic, and what is the consequence of this?
lipophilic, which allows them to cross BBB (effective for brain tumors)
other than brain tumors, what can nitrosoureas treat?
-NHL
-HL
-myeloma
-islet cell carcinoma
-glioblastoma
AEs of nitrosoureas
-N/V
-myelosuppression
-CNS toxic
-pulm. fibrosis
what are platinum analogues for cancer? (not the drugs; just describe them in general)
alkylating-like agents (NOT true alkylating agents) that crosslink DNA and inhibit DNA synthesis
-note the MOA is not entirely clear
what type of proteins do platinum analogues act on?
both cytoplasmic and nuclear
are platinum analogues for cancer cell cycle specific or nonspecific?
Non specific
are platinum analogues used as monotherapy or combotherapy?
Combo therapy b/c they synergize with other drugs:
-alkylating agents
-fluoropyrimidines
-taxanes
cancer types that platinum analogues can treat
-testicular
-ovarian
-bladder
-esophageal
-lung
-head/neck
-colon
-breast
AEs of platinum analogue agents
-N/V
-nephrotoxic
-peripheral neurotoxicity
-myelosuppression
-ototoxicity
platinum analogues used clinically
-Cisplatin (1st gen)
-carboplatin (2nd gen_
-oxaliplatin (3rd gen)
Note the "plat" in the name
what is unique about
-cisplatin
-carboplatin
-oxaliplatin?
-cisplatin requires hydration therapy and premedication; interacts with doxorubicin, rituximab, tacrolimus, topotecan, and aminoglycosides
-carboplatin = thrombocytopenia, and interacts with aminoglycosides
-oxaliplatin = unique neurotoxicity
consider these agents; are they cell cycle specific? are they specific for any type of cancer?
-alkylating agents
-nitrosoureas
-platinum analogues
None of these are cell cycle specific, and all of them treat an array of cancers
which alkylating agents are:
-classic (and the subtypes)
-nonclassic
CLASSIC:
nitrogen mustards:
-cyclophopshamide
-ifosfamide
nitrosoureas:
-carmustine
NON-CLASSIC:
-decarbazine
-temozolomide
This is a duplicate, but it was a separate LO so we are gonna add it.
Explain how cyclophosphamide becomes activated, and which 2 metabolites are its active cytotoxic forms
PRODRUG
1) hepatic CYPs turn it into 4-hydroxxycyclophosphamide, which is at equilibrium w/ aldophosphamide
2) enzymatic activation from 4-OH cyclophosphamide into inactive 4-ketocyclophosphamide
3) aldehyde dehydrog. turns aldophosphamide into inactive metabolite
4) nonenzymatic activity will turn adlophosphamide into toxic metabolites (phosphoramide mustard and acrolein)
again, here is the photo of cyclophosphamide for reference
Which types of cancers are treated by:
-DNA alkylating agents
-nitrosoureas
-platinum analogues
Make a table for this in word
describe the structure of antimetabolites and how this works to treat cancer
structural and chemical analogues of metabolites such as purines and pyrimidines, which are required for normal biological functions such as DNA synthesis
how were antimetabolites determined to be effective cancer agents?
rational synthesis rather than empirical screening
are antimetabolites cell cycle specific or nonspecific?
Specific for S phase (recall this is when DNA synthesis is occurring, so this should make sense)
antimetabolites are most effective for _________
rapidly growing tumors
what limits the use of antimetabolites?
dose-limited myelosuppression
3 major classes of antimetabolites and the representative drug for each class
Folic acid analogues
-methotrexate
pyrimidine analogues
-5-fluorouracil
purine analogues
-6-mercaptopurine
Methotrexate is a ______ analogue
folic acid analogue
MOA of methotrexate
competitive inhibition of DHFR, the enzyme for formation of THF from DHF
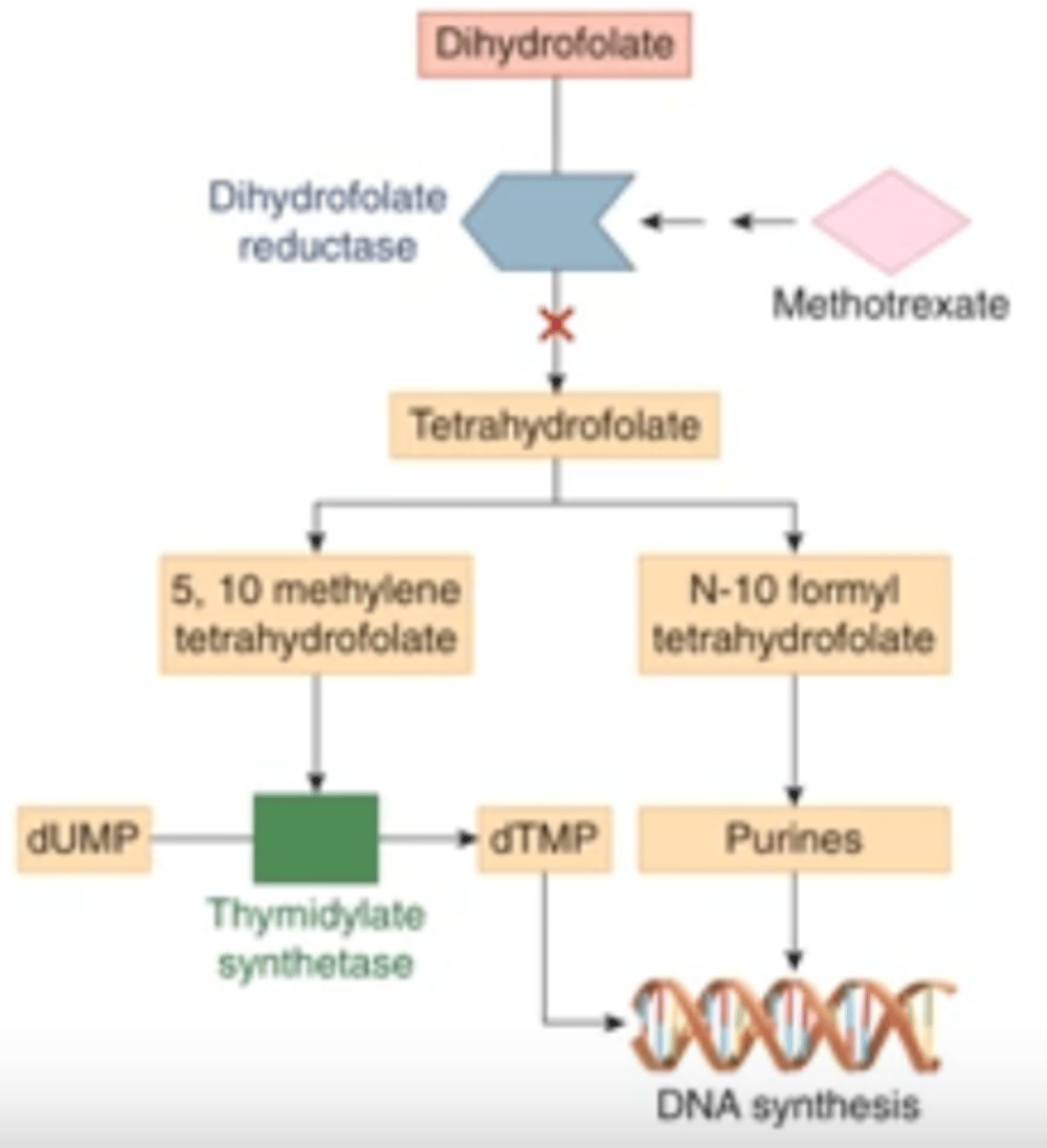
what is the ultimate result of methotrexate inhibiting DHFR?
blocks synthesis of thymidylate and purines, which inhibits DNA synthesis
is methotrexate cell cycle specific or nonspecific?
S phase specific
ROA of methotrexate
PO
IM
intrathecal
uses of methotrexate in cancer
-in combo for ALL
-breast
-cervix
-lung
-head/neck
how does methotrexate in cancer differ in its use for RA?
doses for cancer are much higher
what implications does the higher dose of methotrexate used in cancer have?
may require leucovorin "rescue" (or pretreatment?) to reduce toxicity
which antimetabolite is used to treat only a single cancer?
6-mercaptopurine is for AML only
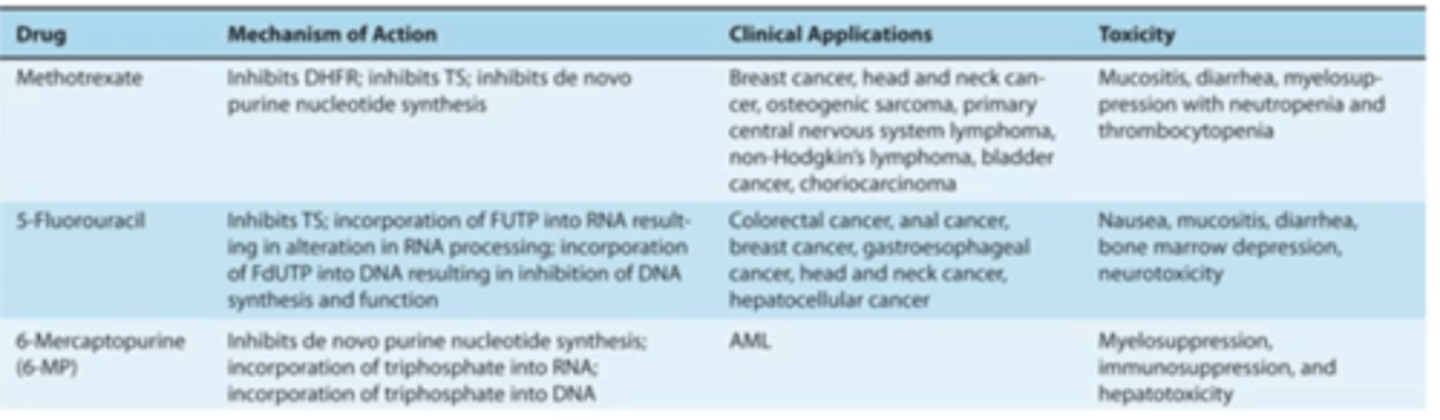
To summarize, what are the MOA, Clinical applications, and toxicity profiles of:
-methotrexate
-5-FU
-6-mercaptopurine
Major classes of natural product anticancer drugs and their representative agents (not exactly a LO, but I'm adding this for reference)
vinca alkaloids:
-vinblastine
-vincristine
taxanes:
-paclitaxel
-docetaxel
antitumor ABX:
-bleomycin
-dexorubicin
-mitomycin
topo 1 inhibitors:
-totopecan
-irinotecan
topoisomerase 2 inhibitors:
-etopside
where do vinca alkaloids come from
periwinkle plant Vinca Rosea
which agents are vinca alkaloids for cancer
-vinblasine
-vincristine
Are vinca alkaloids cell cycle specific or nonspecific?
Specific for M phase (consider MOA to make sense of this)
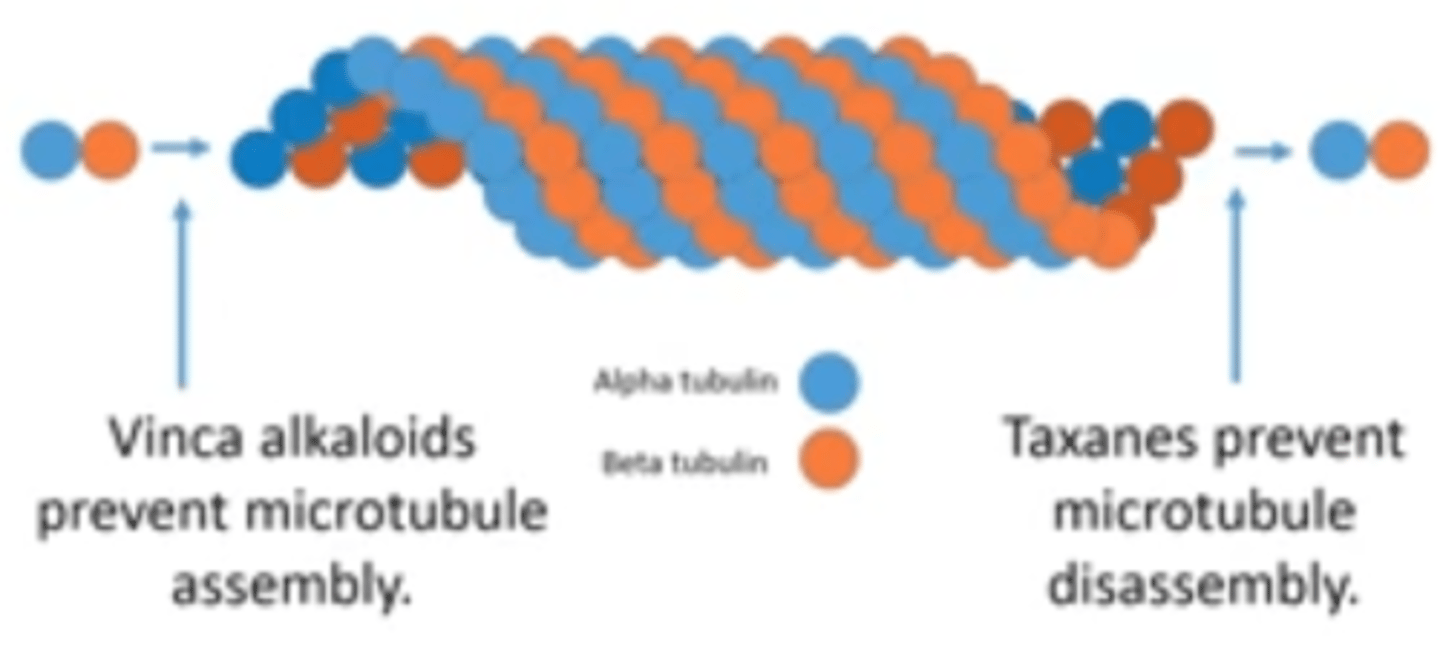
MOA of vinca alkaloids
Promote microtubule disassembly AND prevent Microtubule assembly
ie/ inhibit mitotic spindle assembly by binding tubulin; leads to metaphase arrest
ROA of vinca alkaloids (vinblastine, vincristine)
IV ONLY!!!!
FATAL IF GIVEN ANY OTHER ROUTE; KNOW THIS ***********
VInca = IV
Uses for vinblastine (vinca alkaloid)
ABVD regimen (doxorubicin, bleomycin, vinblastine, decacarbazine) for HL
uses for vincristine (vinca alkaloid)
-CHOP regimen (cyclophosphamide, hydroxydaunoribicin, oncovin, prednisolone) for NHL
-MOPP regimen (mechlorethamine, oncovin, procarbazine, prednisolone) for HL
Oncovin is vincristine
which cancers are AVBD, CHOP and MOPP regimens each used for?
AVBD = HL
MOPP = HL
CHOP = NHL
Other than the MOPP, CHOP, and ABVD regimens, what are vinca alkaloids used to treat?
-solid tumors in children; breast and cervix in adults
-in combo w/ prednisolone for induction and remission of childhood ALL
summarize the uses for vinca alkaloids
-ABVD for HL
-MOPP for HL
-CHOP for NHL
-solid tumors in children
-breast and cervix in adults
-combo w/ prednisolone for ALL
which agents are taxanes
-paclitaxel
-docetaxel
Note the "tax" in the name
where do paclitaxel and docetaxel come from
-paclitaxel is an alkaloid ester from pacific yew tree
-docetaxel is semisynthetic; obtained from european yew tree
are taxanes cell cycle specific or nonspecific?
M phase specific
MOA of taxanes
Promote microtubule assembly and prevent microtubule disassembly
How are taxanes different from vinca alkaloids?
-taxanes PROMOTE assembly (↑ assembly and ↓ disassembly)
-vinca alkaloids PREVENT assembly (↓ assembly and ↑ disassembly)
therapeutic uses for taxanes
Solid tumors:
-NSCLC
-ovarian
-breast
photo of difference b/w MOA of vinca alkaloids and taxanes for reference
-Alkaloids inhibit Assembly
what is another name for antitumor antibiotics?
anthracyclines
prototype anthracyclin, and where does it come from?
Doxorubicin; isolated from streptomyces peucetius
ROA of anthracyclines (antitumor ABX)
IV only!!
-tissue necrosis if not IV
-avoid extravascation when given IV
which agents are anthracyclins?
-doxorubicin (prototype)
-daurobicin
-mitoxantrone
-epirubicin
clinical uses of anthracyclines (antitumor ABX)
-doxorubicin most effective in solid tumors
-daunorubicin most effective against leukemias
major toxicity concern with anthracyclines
cardiotoxic via free radicals
MOA of anthracyclines
Inhibit topo 2:
-blocks DNA transcription and replication
-cause DNA breaks
-high affinity for DNA through intercalation, inhibiting DNA and RNA synthesis
-generation of free radicals
-binds cell membrane to alter fluidity and ion transport
representative antitumor ABX
-mytomycin C
-bleomycin
MOA of mytomycin C
reacts with Gs on complementary DNA strand to cross link DNA (much like alkylating agents)
is mytomycin cell cycle specific or nonspecific
Nonspecific, but maximal cell killing is in G1 and S
therapeutic uses for mytomycin C
-recurrent breast and colon cancers
-metastatic osteogenic cancer
-soft tissue sarcoma
-bladder cancer
AEs of mytomycin C
-myelosuppression
-N/V
-mucositis
-anorexia
-fatigue
-hemolytic uremic syndrome
-extravasation
what is bleomycin and how does it work?
group of glycopeptides that intercalate DNA, generate oxygen free radicals, and cleave DNA
which cell cycle phase(s) are most sensitive to bleomycin?
G0
G2
In class said G2 and M ???
how is bleomycin eliminated?
inactivated in most tissues by bleomycin hydrolase (except skin and lung)
-toxicity in a specific organ occurs due to lack of enzymatic activity in these tissues