Biochem Lec 21- Metabolism: Coenzymes and Energetics
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What does the process of metabolism allow a cell to do?
Extract energy from its environment
Synthesize the chemical building blocks of macromolecules
Briefly describe metabolic pathways.
Metabolic pathways are coupled and interconnected
They contain many recurring elements including intermediates and modes of regulation
Metabolic pathways are basically sequences of chemical reactions that convert a particular molecule into another in a carefully defined way
Define catabolism.
Catabolism→ reactions that break down complex molecules into simpler ones to capture energy in useful forms
Oxidation of carbon-rich “fuel” molecules powers the formation of ATP (e.g., glucose and fatty acids)
Define anabolism.
Anabolism→ reactions that construct a more complex molecule from simpler molecules by using energy
What are forms of “useful” energy?
Chemical
ATP and other high energy phosphates
Thioesters (Co-enzyme A)
Ionic
Proton and ion gradients
Reducing power
Nicotinamide (niacinamide) and Flavin co-enzymes
Chemical energy “currency” in the cell is phosphate based. Explain how ATP fits into this.
ATP acts as the free-energy donor in most energy-requiring processes
ATP→ a nucleotide consisting of adenine, ribose, and a triphosphate unit
ATP acts as an energy carrier because the hydrolysis of its phosphoanhydride bonds is very exergonic→ -30.5 kJ/mol
ATP is a “phosphoryl group donor”→ has high “phosphoryl transfer potential
Why is the hydrolysis of ATP’s phosphoanhydride bonds very exergonic. Why is this?
Pi has greater resonance stabilization than ATP phosphoryl groups
Electrostatic repulsion of the triphosphate makes ATP less stable→ donation of phosphoryl group makes products more stable than reactants
Entropy increases when Pi is liberated
ADP and Pi can form new interactions with water
What do reactions using energy coupling often involve and why?
They often involve the hydrolysis of ATP→ ATP has a very negative free energy that can lower the overall free energy of a typically unfavorable reaction when they are energetically coupled
What determines whether a reaction will occur spontaneously? How does energetic coupling fit into this?
The standard biological free energy (ΔGº”)
The relative concentrations of substrates [A] and products [C] and [D]
The overall ΔG for a chemically coupled series of reactions equals the sum of the ΔG of the individual steps:
A→ B + C ΔGº’= 21 kJ/mol
B→ D ΔGº’= -34 kJ/mol
Overall ΔGº’= (21 + -34)= -13 kJ/mol
What are 3 enzymes that use ATP to drive unfavorable reactions?
Kinases→ phosphotransfer
ROH + ATP→ RO-(PO3-) + ADP
Carboxylases→ addition of CO2
R-CH3 + CO2 + ATP→ RCH2-COO- + ADP + Pi
Synthetases→ ligation of two substrates
A + B + ATP→ A-B + ADP + Pi
Describe the ATP cycle.
ATP→ ADP
Motion
Active transport
Biosyntheses
Signal amplification
ADP→ ATP
Oxidation of fuel molecules
Photosynthesis
What is the energy status of any given cell manifested by?
Adenylate Energy Charge
AEC fluctuates from 1 (all ATP) to 0 (all AMP… death)
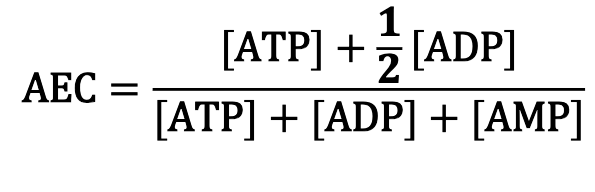
What is Co-Enzyme A?
Co-Enzyme A is a chemical form of useful energy:
Derived from the dietary vitamin pantothenic acid
Stores energy in the form of an activated thioester bond
Contains a thiol group that carries activated acyl groups in a thioester linkage
“Activated carrier”→ the thioester is a high energy bond that can be used for biosynthesis or for the production of energy
Vitamin→ Pantothenic acid
Coenzyme→ Coenzyme A
Typical reaction type→ Acyl group transfer
Consequences of deficiency→ Hypertension
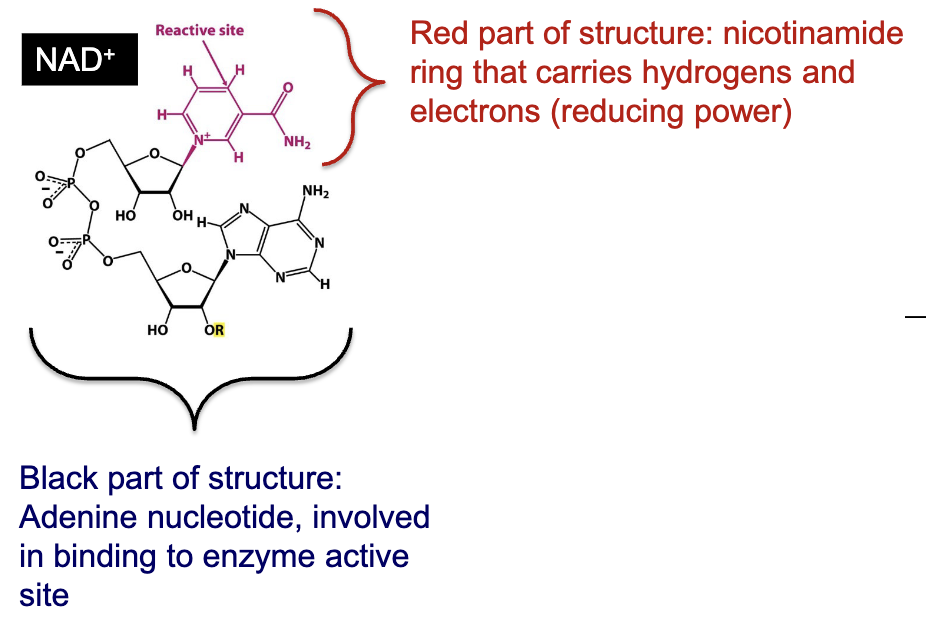
What are NADH/NADPH? Describe their structure and what they do.
NADH and NADPH are “reducing power” forms of useful energy:
Activated carrier
R= H→ nicotinamide adenine dinucleotide (NAD+)→ catabolism and energy production
R= phosphate→ nicotinamide adenine dinucleotide phosphate (NADP+)→ reducing power for biosynthesis
Contains an adenine nucleotide involved in binding to enzyme active site and a nicotinamide ring that carries hydrogens and electrons (reducting power)
Serve as two electron acceptors in oxidation-reduction reactions
Enzymes that catalyze these reactions and that use NAD+ or NADP+ as a coenzyme are called “dehydrogenases” or “oxidoreductases”
Vitamin→ Nicotinic acid
Coenzyme→ Nicotinamide adenine dinucleotide (NAD+)
Typical reaction type→ Oxidation-reduction
Consequences of deficiency→ Pellagra (dermatitis, depression, diarrhea)
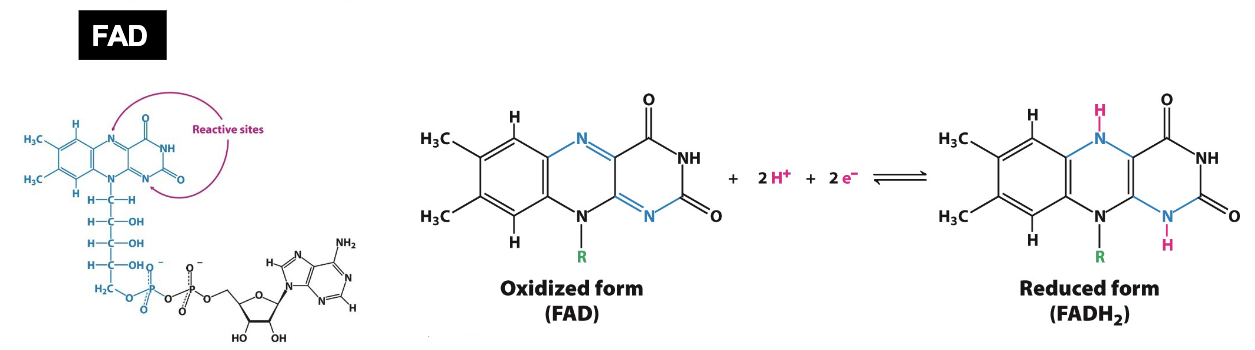
What is FADH2?
FADH2 is a “reducing power” form of useful energy:
Activated carrier
Serves as a two electron acceptor for dehydrogenases and oxidoreductases
It generally has less reductive power than NAD+
FAD (flavin adenine dinucleotide) is tightly (often covalently) bound to enzymes (“flavoproteins'“)
Vitamin→ Riboflavin (B2)
Coenzyme→ Flavin adenine dinucleotide (FAD)
Typical reaction type→ Oxidation-reduction
Consequences of deficiency→ Cheilosis and angular stomatitis (lesions of the mouth), dermatitis
Summarize activated carriers.
Carbon fuel oxidation is performed in metabolism through the help of “activated carriers”
ATP is an activated carrier of phosphoryl groups because phosphoryl transfer from ATP is an exergonic process
Metabolism also uses other activated carriers for fuel oxidation, which are derived from vitamins (coenzymes)→ REDUCING POWER
