HTHSCI 2HH3 - Lecture: Antimicrobials
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
Prophylactic Therapy - Antimicrobial Therapy
- Given to prevent an infection
- Consider in individuals at increased risk of developing infection
- Infection that has not occurred
Empiric Therapy - Antimicrobial Therapy
- Treatment given when an infection is suspected, before the organism(s) is identified
- Antimicrobial selection guided by patient’s presentation and history
Targeted Therapy - Antimicrobial Therapy
- Treatment selected to target a specific organism known to be causing an infection
- Antimicrobial selection guided by organism culture results and sensitivities
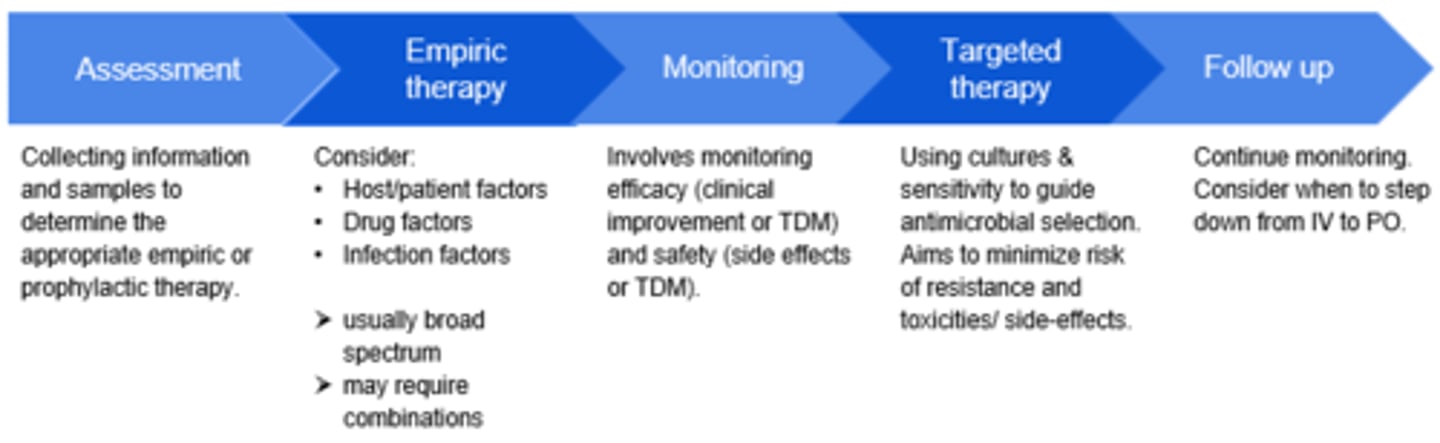
Steps to Antimicrobial Therapy
1. Assessment
2. Empiric Therapy
3. Monitoring
4. Targeted Therapy
5. Follow Up
Assessment - Steps
1. Is there an infection?
- History of presenting illness
- Clinical signs and symptoms
- Source of infection?
- Non-infectious causes
2. Any infectious history?
- Comorbidities
- Recent travel
- Sick contacts
- Recent hospitalization
- Immunization history
Assessment - Sample Collection
Sample types:
- Source: blood, urine, sputum, cerebrospinal fluid (CSF - lumbar puncture), biopsy, nasal/rectal swab
- Purpose: clinical (from presumed site of infection) or surveillance/screening (ex. MRSA swab)
Tests
- Gram staining (<24h)
- Organism identification - culture (usually 24 to 48h), polymerase chain reaction (PCR)
- Antibiotic susceptibility (>48h)
*Draw a culture prior to administering any antimicrobial agents
*Collecting samples after a single dose of antimicrobials can reduce the likelihood of isolating an organism
Empiric Therapy
How to select appropriate empiric therapy?
Host/patient factors:
- Past medical history
- Age
- Allergies
- Prior infections or hospitalization
- Prior antimicrobial history
- Prior drug colonization
- History of resistance
Drug factors:
- Pharmacokinetics (PK) - The movement of drugs within the body
- Absorption: Route of administration (IV vs PO)
- Distribution: Will the drug reach the site of infection?
- Metabolism: How is the drug broken down? Drug-drug interactions
- Excretion: How does the body get rid of the drug?
- Pharmacodynamics (PD): The action of the drug in the body
- Drug spectrum of activity, mechanism of action, combination therapy, resistance
Infection factors:
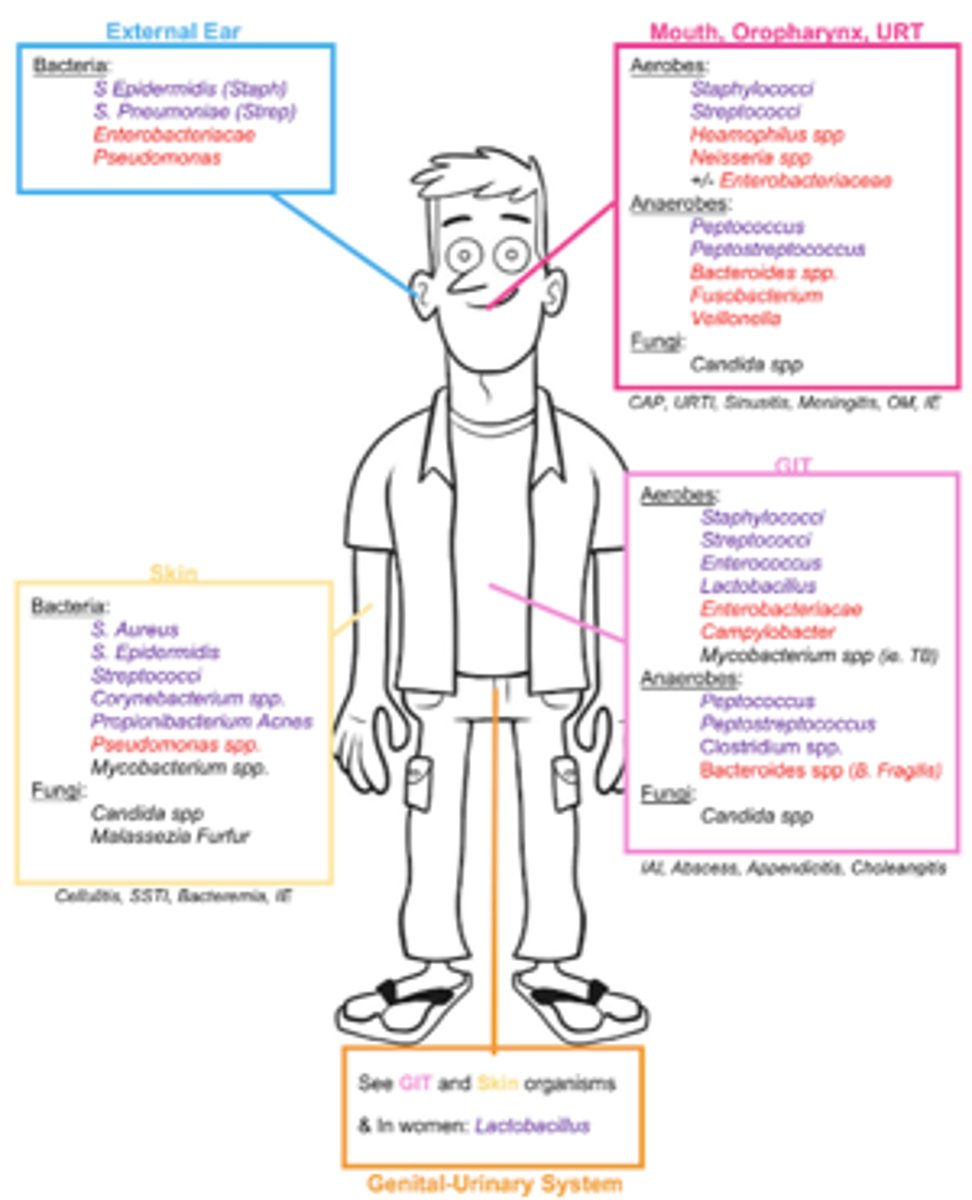
- Many infections are caused by organisms that are a part of our own normal flora
- If we know the typical flora for each organ site, we can often predict the likely causative pathogens of infection
Spectrum of Drug Activity
Broad Spectrum (used for empiric therapy - don't yet know what targeting against):
- Active against a large variety of organisms
- Good: Increased likelihood of activity against an unknown organism
- Bad: Promote the development of drug resistance. Place in therapy: Consider for empiric therapy prior to organism identification
Narrow Spectrum (for more targeted therapy):
- Active against limited groups of organisms
- Good: Associated with reduced development of drug resistance
- Bad: Risk of poor activity/lack of effect against an unknown organism. Place in therapy: Often used for targeted therapy after organism has been identified

Drug Factors - Mechanism of Action #1
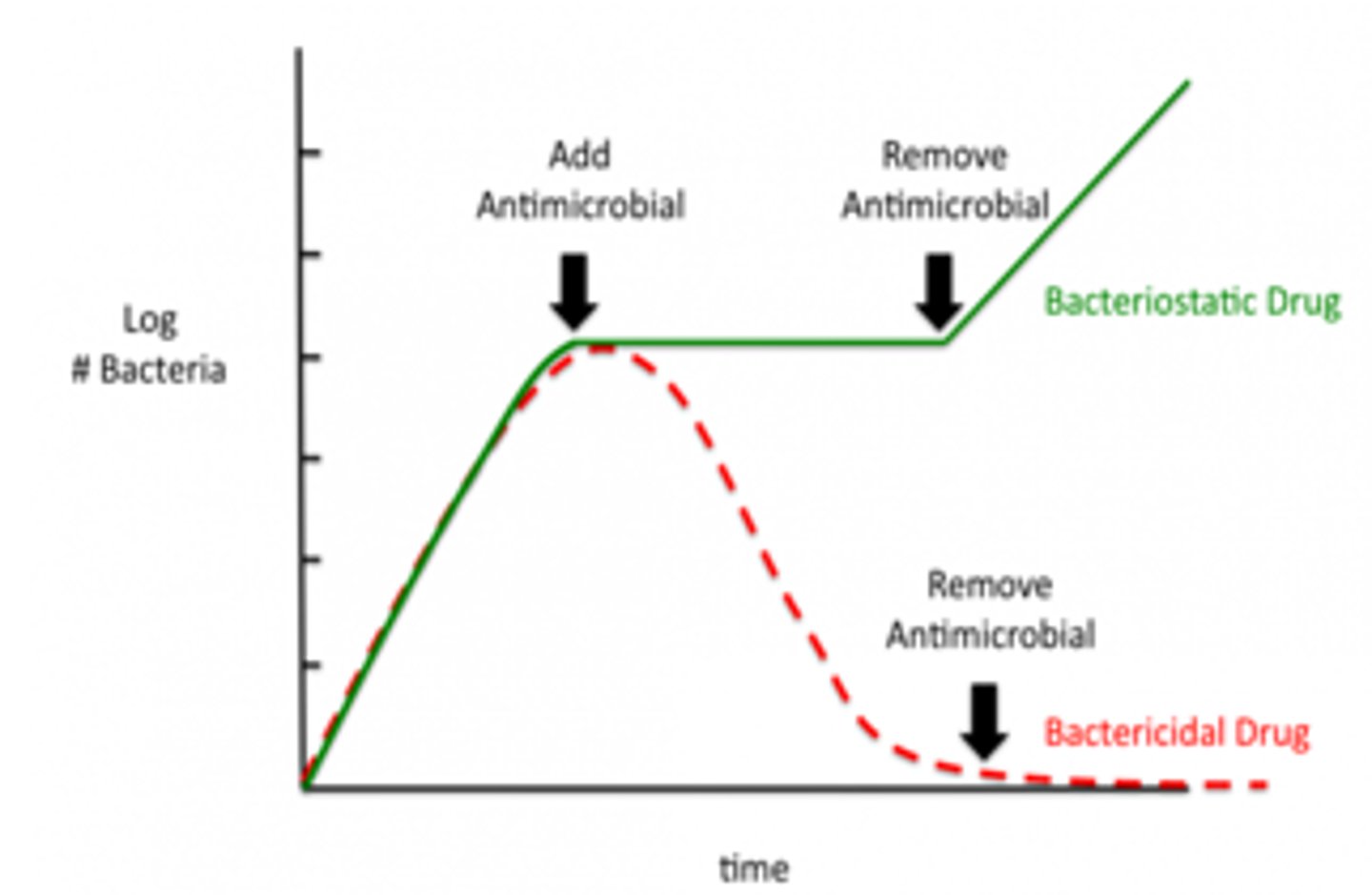
Bacteriostatic:
- Inhibits/slows bacterial growth (to create static phase - plateau)
- Requires a functioning immune system to clear infection
- Used in less serious infections
Bactericidal:
- Causes bacterial death (killing it off)
- Preferred in serious infections or immunocompromised hosts
Drug Factors - Mechanism of Action #2
Time-dependent Killing:
- Drug concentration must remain above minimum inhibitory concentration (MIC) for effect.
- The absolute amount above MIC does not impact activity
Concentration - Dependent Killing:
- The peak drug concentration determines effect
- Demonstrate a post-antibiotic effect where activity continues even when drug concentration is less than the MIC
*MC = Lowest concentration of drug needed to inhibit bacterial growth
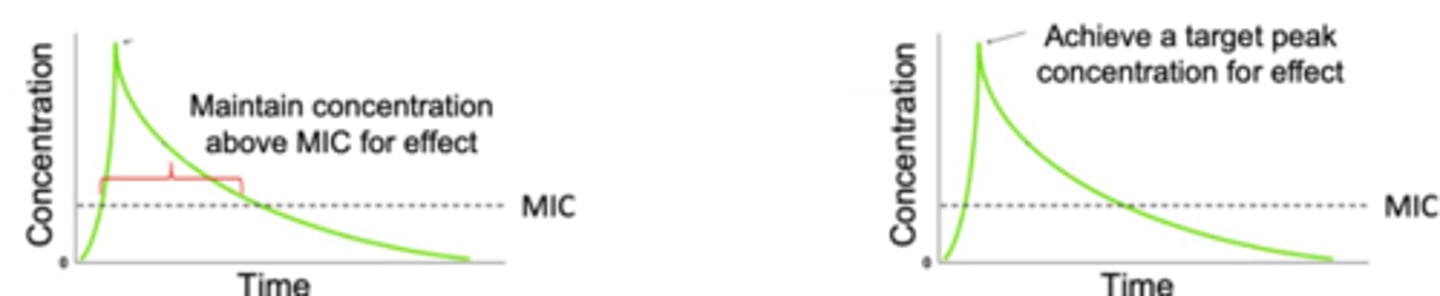
Synergism - Combination Therapy
- Combining different antimicrobials to produce an effect that exceeds the sum of their individual effects
Broadens Spectrum - Combination Therapy
- Combining antimicrobials with different spectrum to fill gaps in coverage
Double Coverage - Combination Therapy
- Combining 2 different antimicrobials with activity against the same organism of interest
- Increases likelihood of success
- Reduces development of antimicrobial resistance
Antimicrobial Resistance
- The ability of certain organisms to develop a tolerance to specific antimicrobials to which they were once susceptible
- This process occurs naturally over time, but is accelerated by the misuse and overuse of antimicrobials
Risk Factors for Developing Drug Resistant Organisms
- Prior antibiotic exposure
- Underlying disease (e.g. hemodialysis)
- Prior hospitalization
- Invasive procedures in healthcare settingsg
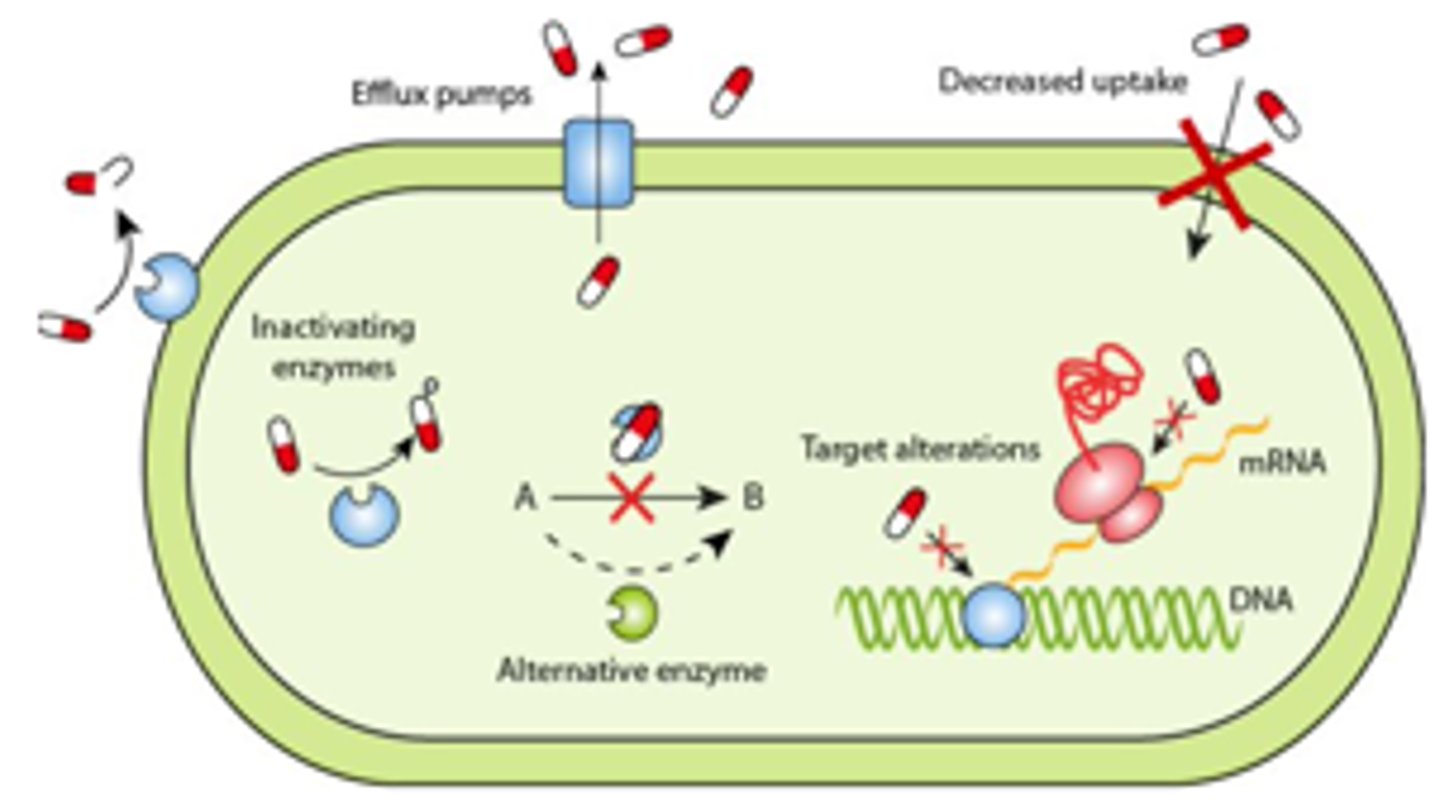
Drug Factors - Mechanisms of Resistance
1. Preventing the antimicrobial from reaching its target at sufficient concentrations:
- Decreased uptake
- Inactivating enzymes (ex. beta-lactamases)
- Increased efflux
2. Modifying the target of the antimicrobial:
- Altering the target
- Alternative enzymes
Site of Infection
- Many infections are caused by organisms that are a part of our own normal flora
- If we know the typical flora for each organ site, we can often predict the likely causative pathogens of infection
Monitoring - Steps
Therapeutic Drug Monitoring (TDM)
- Measuring drug levels in the blood can help guide dosing to ensure efficacy & safety
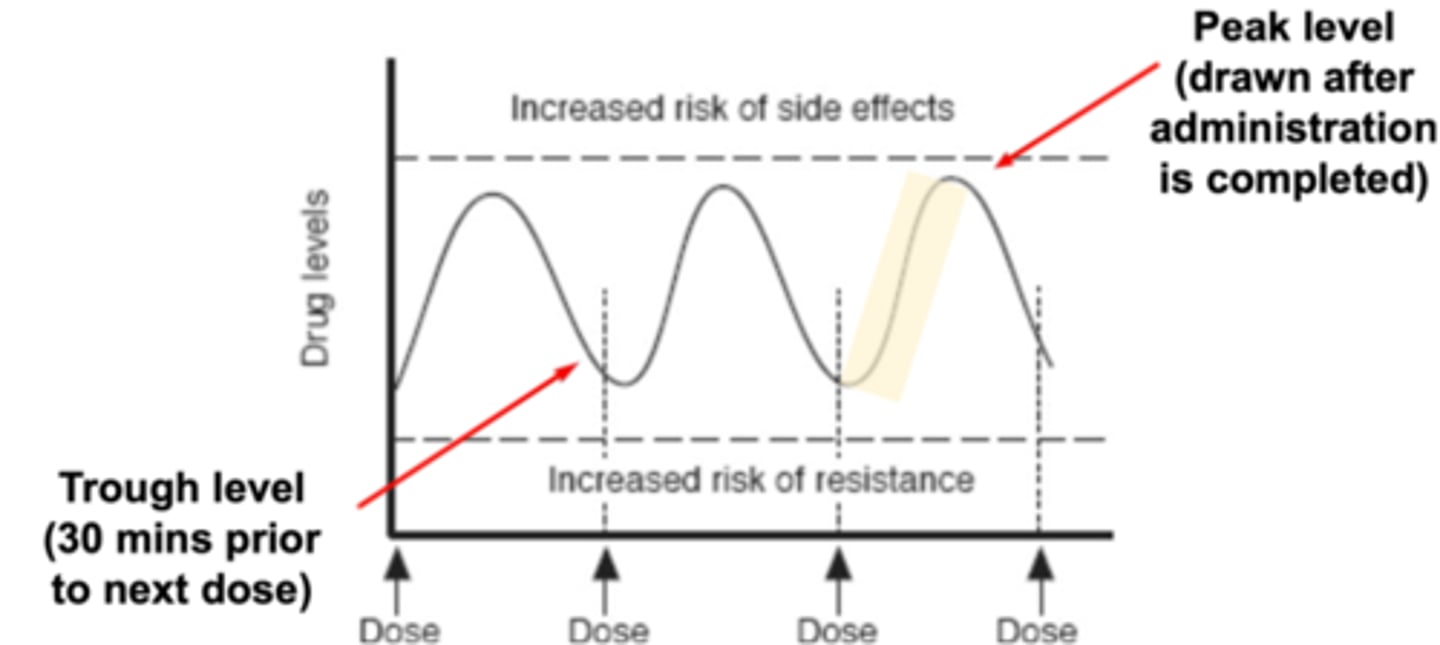
- Timing of blood collection is an essential component of TDM (may want to target antibiotic to trough level or peak level)
Different antimicrobials require levels to be drawn at various time points:
- Trough level (lowest serum concentration) = draw blood 30 mins prior to next dose (draw blood before giving dose)
- Peak level (highest serum concentration) = draw blood immediately after the dose has been administered (wait for full infusion to be administered before drawing blood)
*Steady state = Stable levels of antimicrobial in the body
Targeted Therapy - Steps
- Modifying the antimicrobial regimen based off culture & sensitivity results
- Frequently involves switching from broad -> narrow spectrum agents (vice versa depending on situation)
- Minimize the risk of developing antimicrobial resistance
- Reduce risk of toxic side effects
- Choose an antimicrobial that has demonstrated activity against the organism but has the narrowest spectrum and the least toxicity
Follow-Up Steps
Continue to monitor for symptom resolution, side effects and therapeutic drug levels if required:
Assess for opportunities to switch from IV → PO:
- Clinical improvement?
- Tolerating other oral meds/ foods?
*Average duration of therapy:
- Pneumonia, skin infections: ~5-10 days
- Bloodstream infections: Bacterial: ~1-2 weeks; Fungal: ~2 weeks to months
- Meningitis: ~1-2 weeks
Antimicrobial Principles
Antibiotic Targets
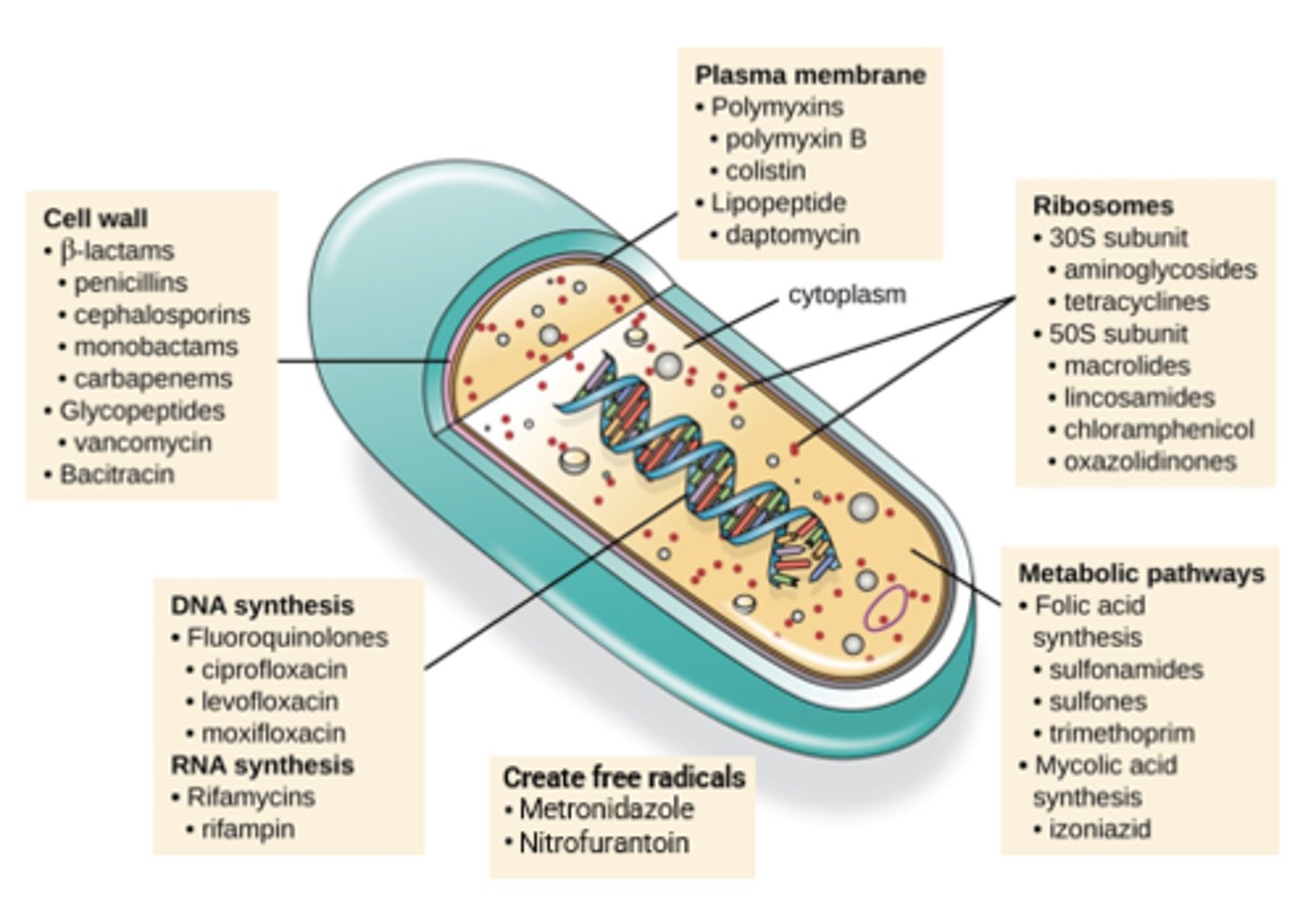
Cell Wall Synthesis
- Beta-lactams
*Dont need to know sub-groups
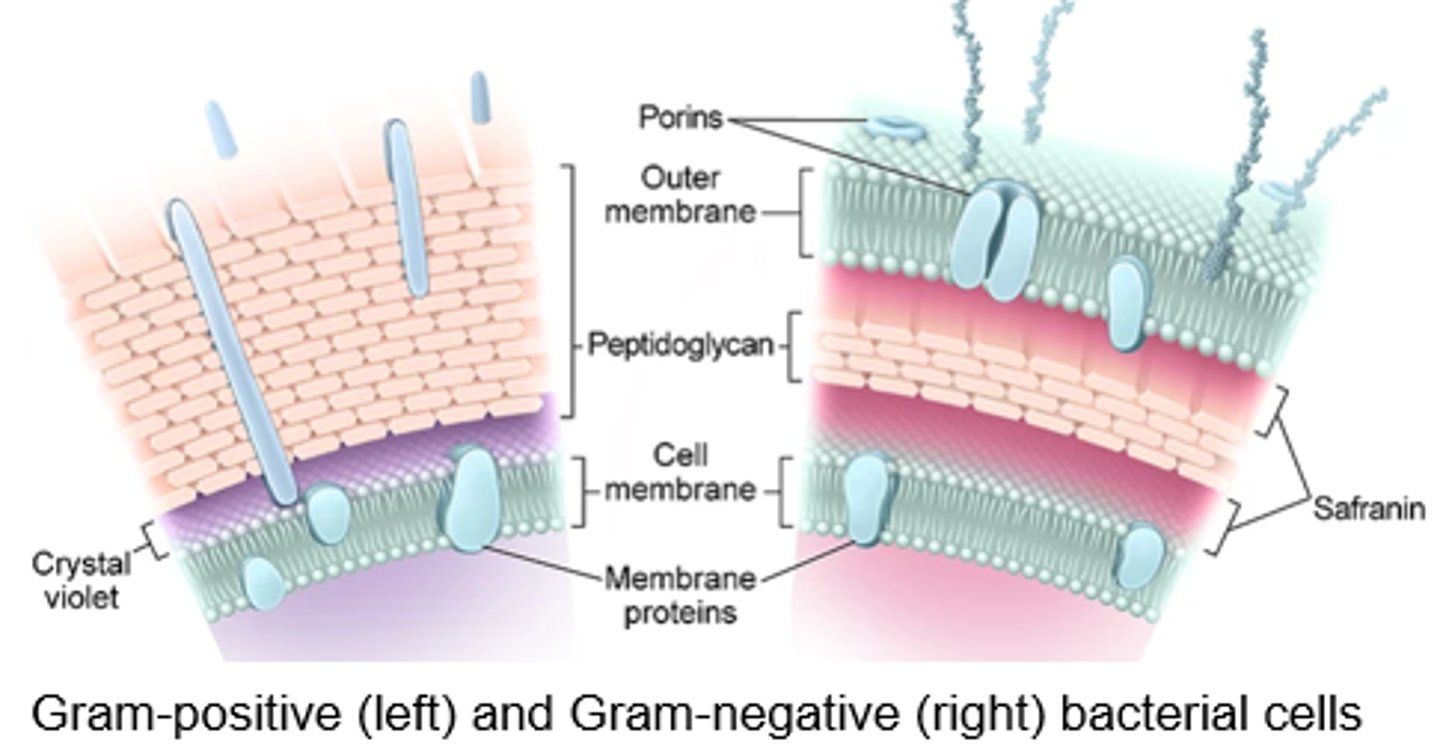
Bacterial Cell Wall
- Peptidoglycan: major component
Transpeptidation:
- The last step of cell wall synthesis
- Penicillin binding proteins (PBPs) form cross links in the cell wall
Beta-Lactams - Mechanism of Action
Beta-lactams interrupt cell wall synthesis by:
- Binding to PBPs
- Inhibits transpeptidation
- Results in: Improper cell wall formation → inability to withstand osmotic pressure → cell ruptures → cell death
- Bactericidal (cell dying), time-dependent killing
Beta-Lactams - Mechanisms of Resistance
1. Bacterial cell production of beta-lactamases
2. Modification PBP binding site (ex. Methicillin-resistant Staphylococcus aureus [MRSA] - makes it difficult for penicillin to bind to, making it not active)
3. Changes to porin channels
4. Drug efflux pumps
![<p>1. Bacterial cell production of beta-lactamases</p><p>2. Modification PBP binding site (ex. Methicillin-resistant Staphylococcus aureus [MRSA] - makes it difficult for penicillin to bind to, making it not active)</p><p>3. Changes to porin channels</p><p>4. Drug efflux pumps</p>](https://knowt-user-attachments.s3.amazonaws.com/e971d2f0-eaef-4483-adde-d7041c24ada1.jpg)
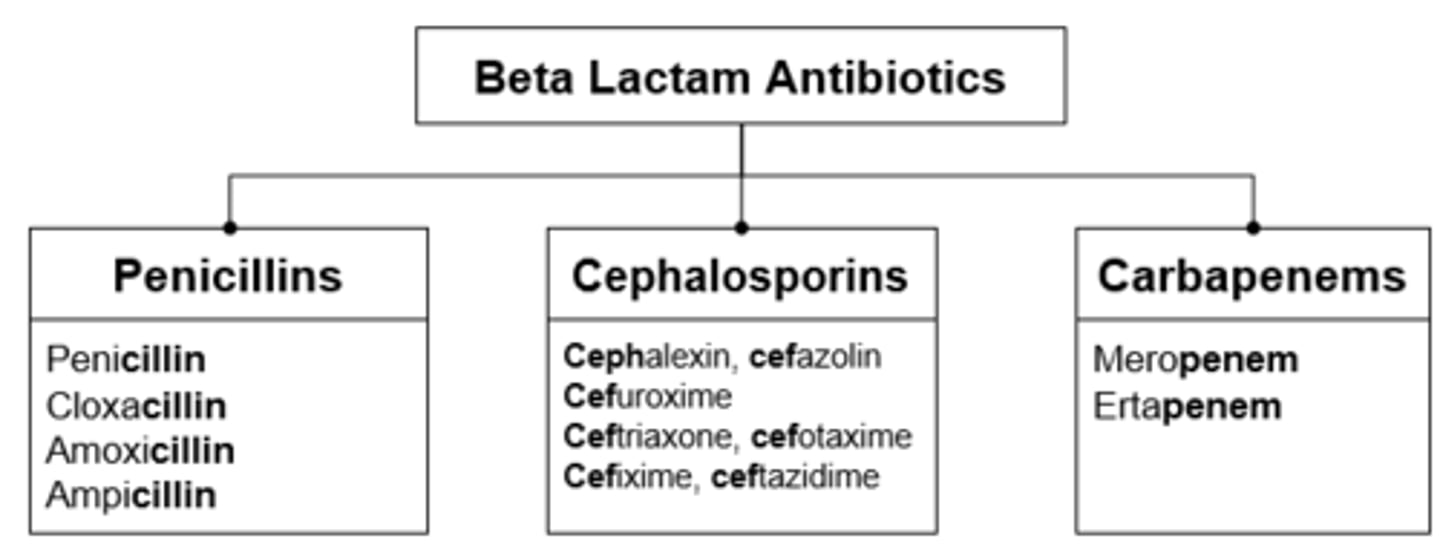
Beta-Lactams - Penicillin's
GI Upset = Nausea, vomiting, diarrhea
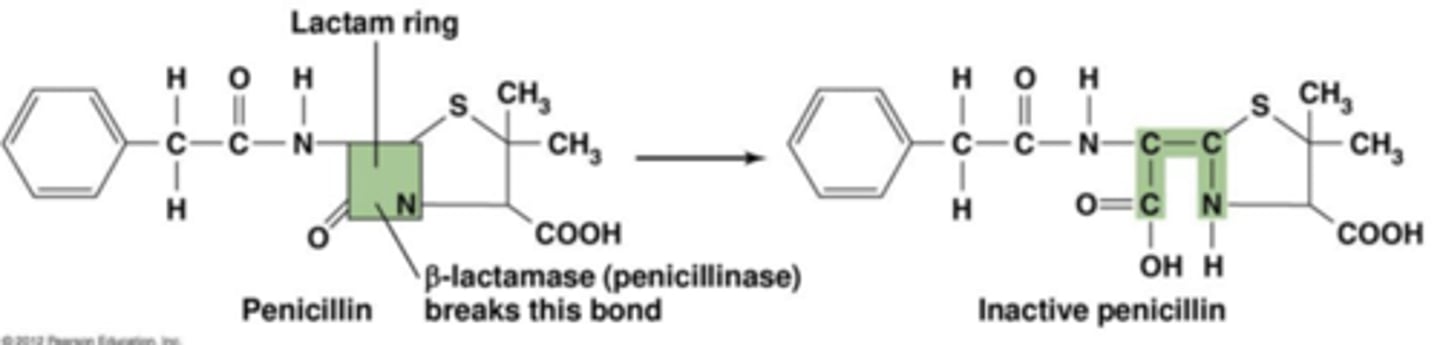
Beta-Lactamase
- Some bacteria can produce beta-lactamases
- Beta-lactamase: An enzyme that cleaves the beta-lactam ring through hydrolysis
- Inactivates beta-lactam antibiotics
*Green – Hydrolyze beta-lactamase which inhibits/makes penicillin inactive
Beta-Lactamase Inhibitors
Some beta-lactams are paired with beta-lactamase inhibitors to overcome resistance:
- Inactive beta-lactamases to extend spectrum of activity → MSSA, gram negatives, anaerobes
- Increase risk of nausea, vomiting and diarrhea (including C. difficile infections)
Examples of beta-lactams beta lactamase-inhibitors:
- Amoxicillin-clavulanic acid (IV, PO)
- Piperacillin-tazobactam (IV)
Beta-Lactams - Cephalosporins
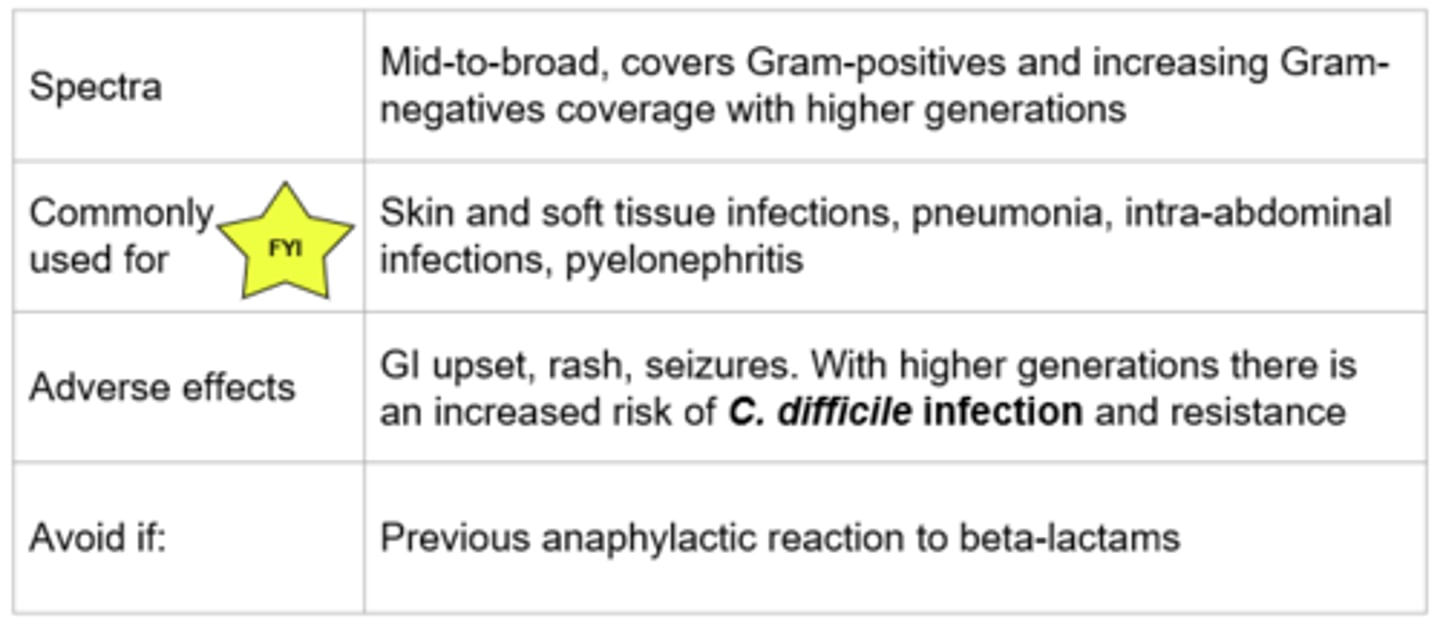
Generations of Cephalosporins
Further generation = More broad to apply to more conditions
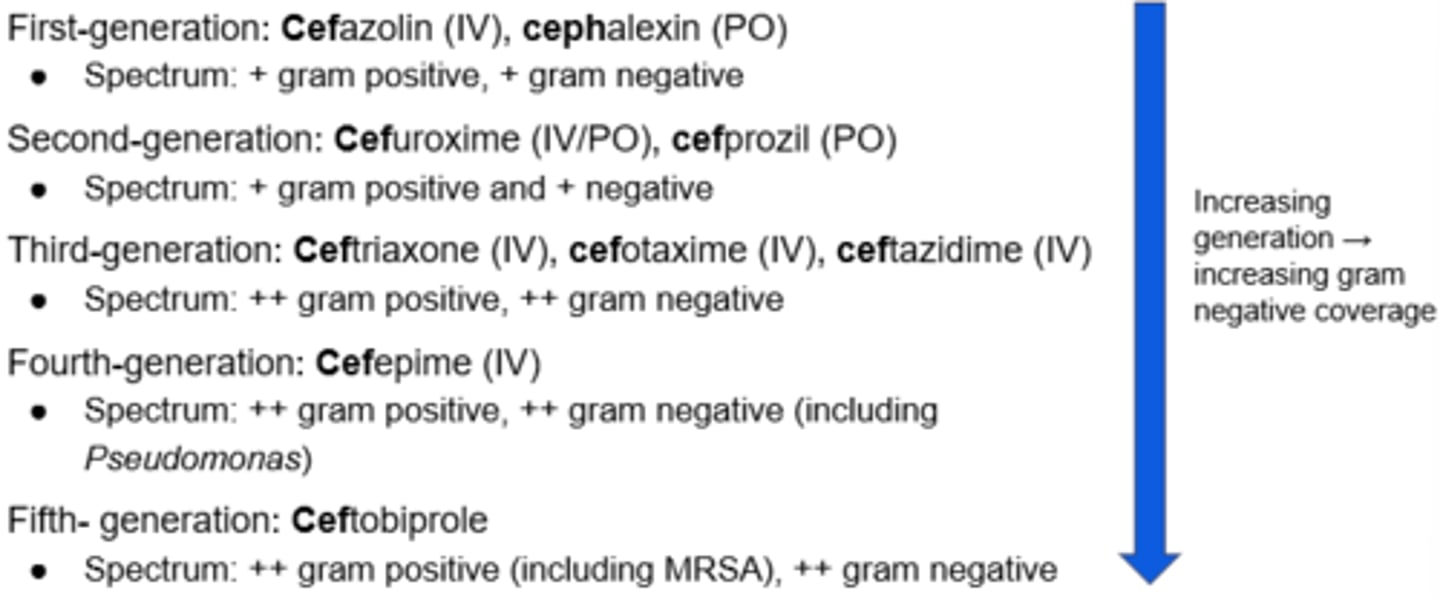
Beta-Lactams - Carbapenems

Beta-Lactam Allergies
Up to 10% of patients have a reported penicillin allergy:
- < 1% are a true allergy
- Many outgrow true allergy
*True penicillin allergies:
- Type I IgE-mediated: Anaphylaxis, hypotension, angioedema
- Avoid all penicillins and cephalosporins with similar side chain
- Cross-reactivity between beta-lactams:
- ~ 1% between penicillins and cephalosporins
- ~ 0.1% between penicillins and carbapenems
Interactions of Beta-Lactams
Don't use the star ones with each other - use open spaces!
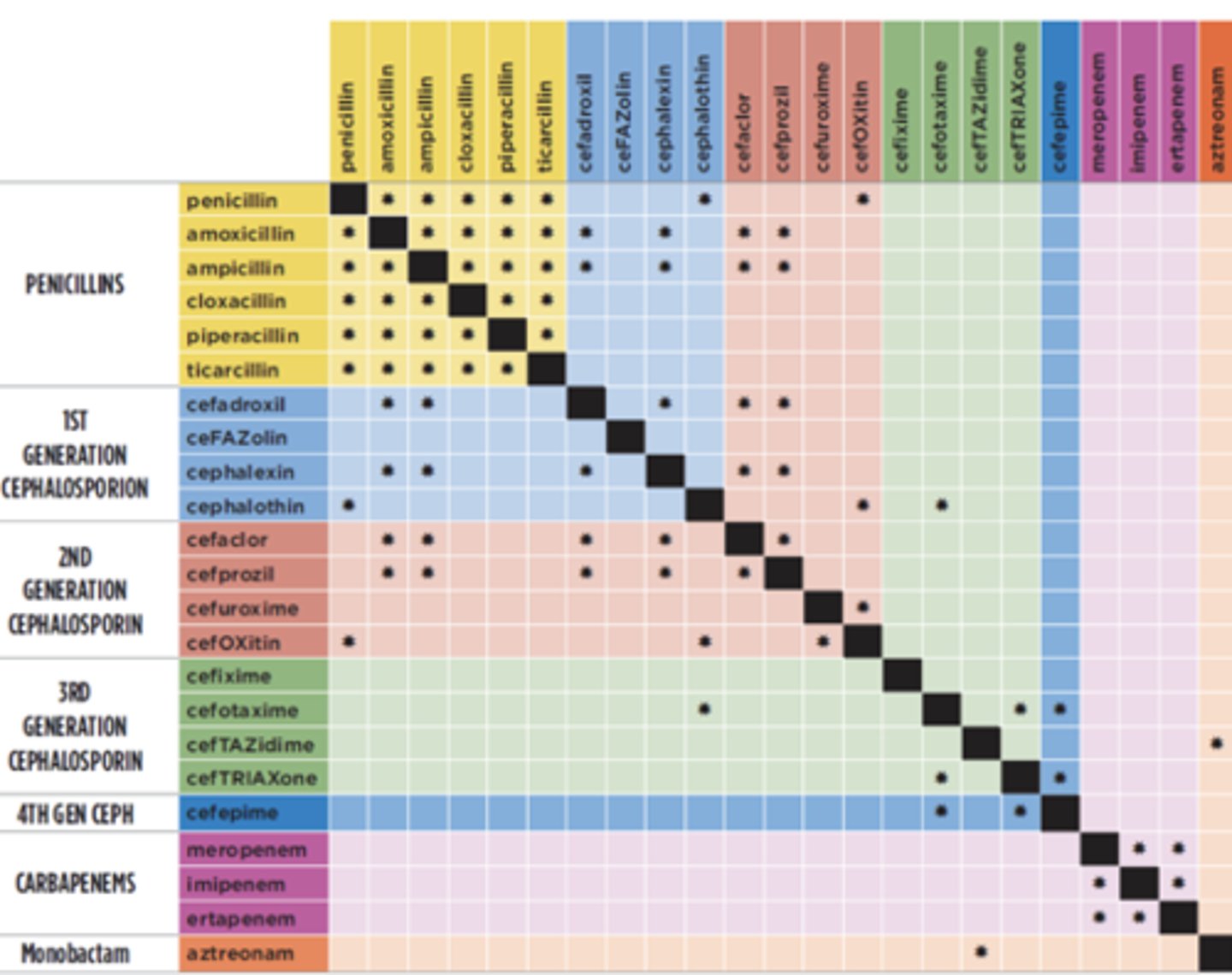
Severe Non-IgE Mediated Hypersensitivity Reactions
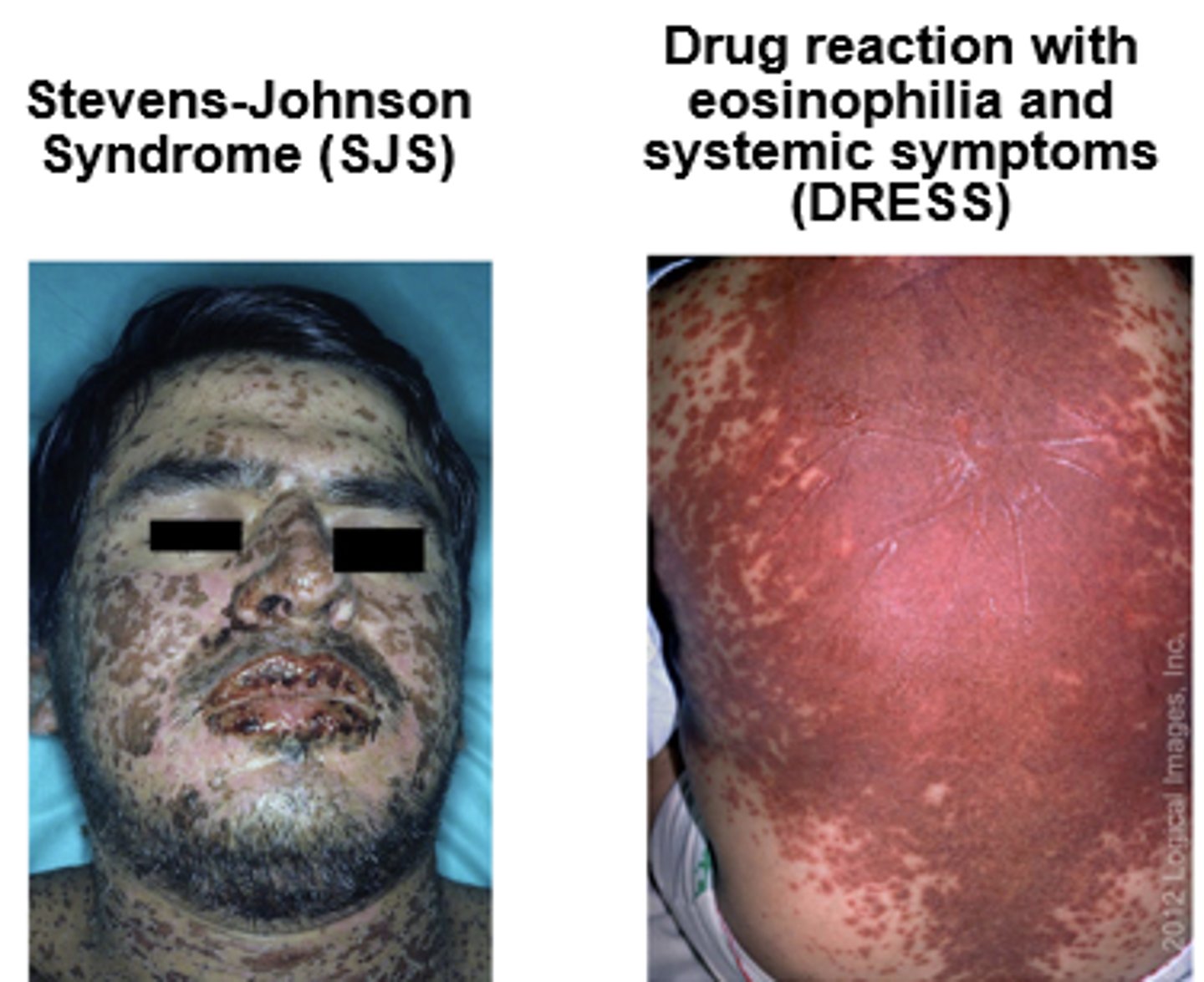
- Type IV non-IgE-mediated: Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug rash with eosinophilia and systemic symptoms (DRESS)
- *Avoid all beta-lactams (penicillins, cephalosporins and carbapenems)
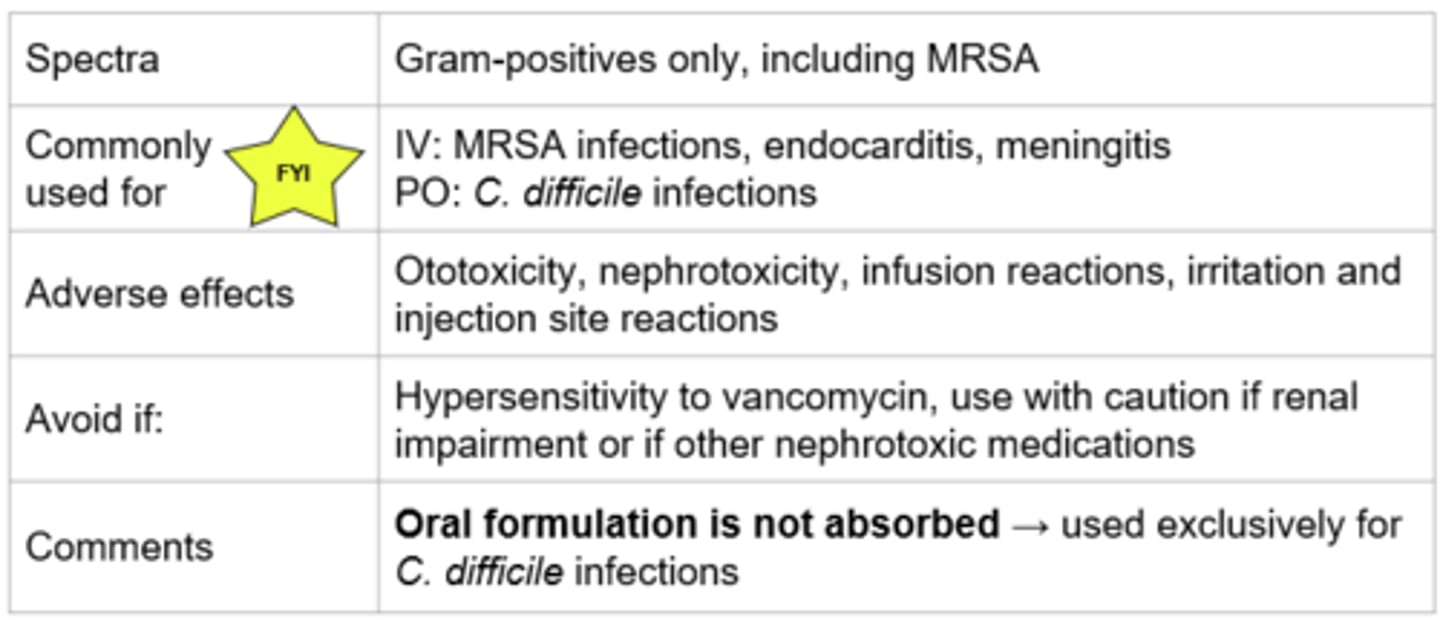
Vancomycin - Mechanism of Action
- Inhibits cell wall synthesis
- Binds to the terminal end of peptidoglycan precursor → prevents polymerization → weakens the cell wall → cell death
- Bactericidal, time-dependent killing
*Cell wall synthesis
Vancomycin
Vancomycin - Infusion Reaction (Flushing Syndrome)
- Characterized by pruritus, flushing, and erythema of the face and upper torso → NOT life-threatening + NOT an allergy
- Managed by slowing the infusion rate
Due to rapid infusion of the drug leading to histamine release:
- Related to infusion rate
Vancomycin allergy:
- Anaphylaxis, hypersensitivity, hives, angioedema, bronchoconstriction → can be life-threatening
- Managed by stopping infusion and administering epinephrine
Vancomycin - Monitoring
Nephrotoxicity:
- Monitor for increasing serum creatinine (SCr) and decreasing urine output
Therapeutic drug monitoring:
- Serum trough levels once at steady state (pre-fourth dose): Most infections → Target = 10-15 mg/L; MRSA and CNS infection → Target = 15-20 mg/L or AUC/MIC of 400-600 mg*h/L
- Low trough (< 10 mg/L) → subtherapeutic, inadequate dose for treatment
- High trough (> 20 mg/L) → supratherapeutic, increased risk of nephrotoxicity
*If trough is out of range, let pharmacist know for dose adjustment
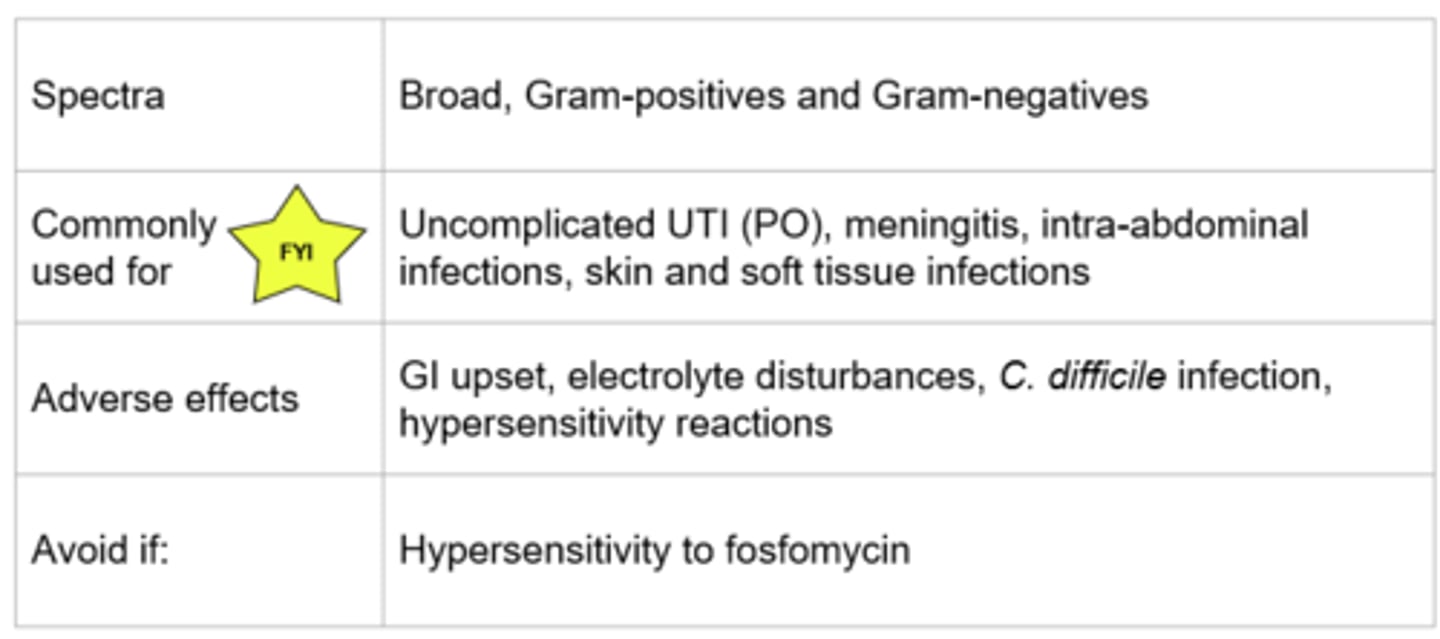
Fosfomycin - Mechanism of Action
- Inactivates MurA enzyme involved in peptidoglycan synthesis → weakens cell wall → cell lysis
- Also inhibits adherence of bacteria to epithelium
- Bactericidal, concentration dependent killing
*Cell wall synthesis
Fosfomycin
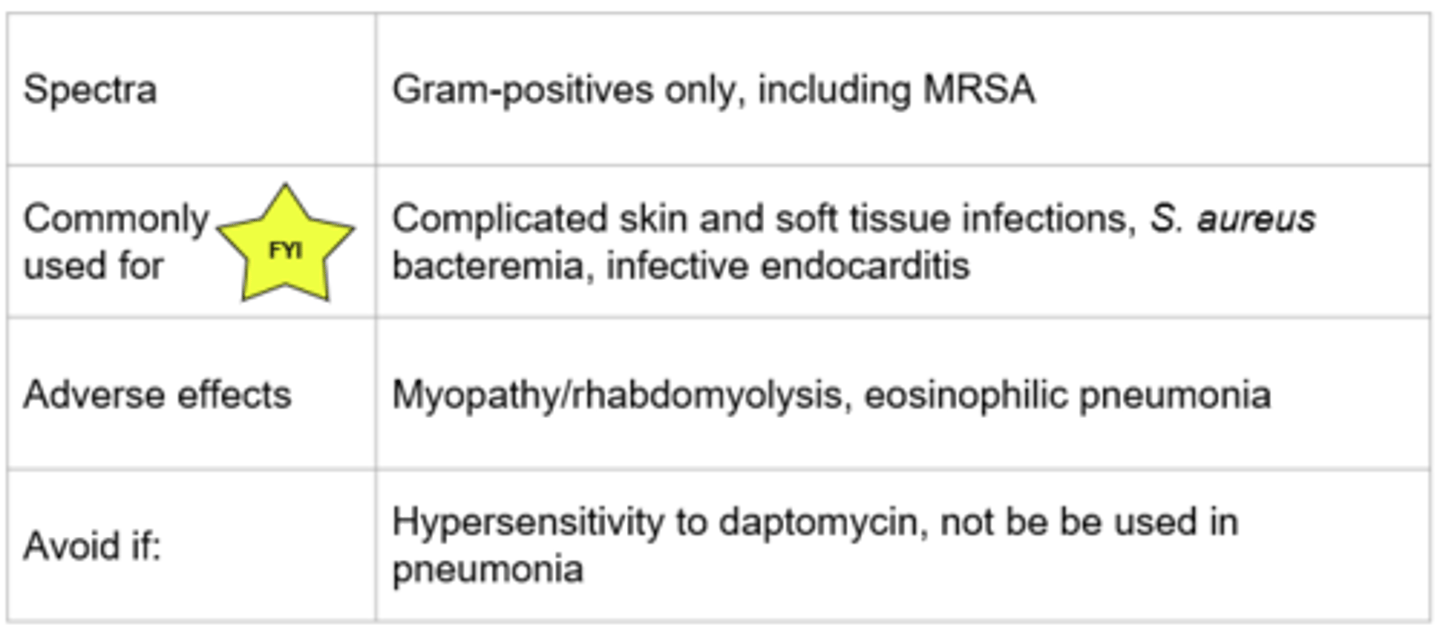
Daptomycin - Mechanism of Action
- Binds to the cell membrane leading to depolarization, efflux of potassium, and inhibition of DNA, RNA and protein synthesis
- Bactericidal (cell death), concentration dependent killing
*Plasma membrane disruption
Daptomycin
Eosinophilic pneumonia (due to lung surfactant inactiviating)
Daptomycin - Monitoring
Rhabdomyolysis: Rapid breakdown of skeletal muscle
- Can be life-threatening
- Monitor: patient reported muscle pain, creatine kinase (CK if it is rising) and dark urine
Eosinophilic pneumonia:
- Monitor: eosinophils (if levels are rising), new-onset fevers and dyspnea
Fluroquinolones - Mechanism of Action
- Inhibition of DNA synthesis by inhibiting bacterial DNA gyrase and topoisomerase IV → promotes the breakage of DNA
- Bactericidal (cell death), concentration dependent killing
- Examples: Ciprofloxacin, levofloxacin, moxifloxacin
*DNA synthesis
Fluroquinolones
C. Difficile Infections - Due to being broad-spectrum
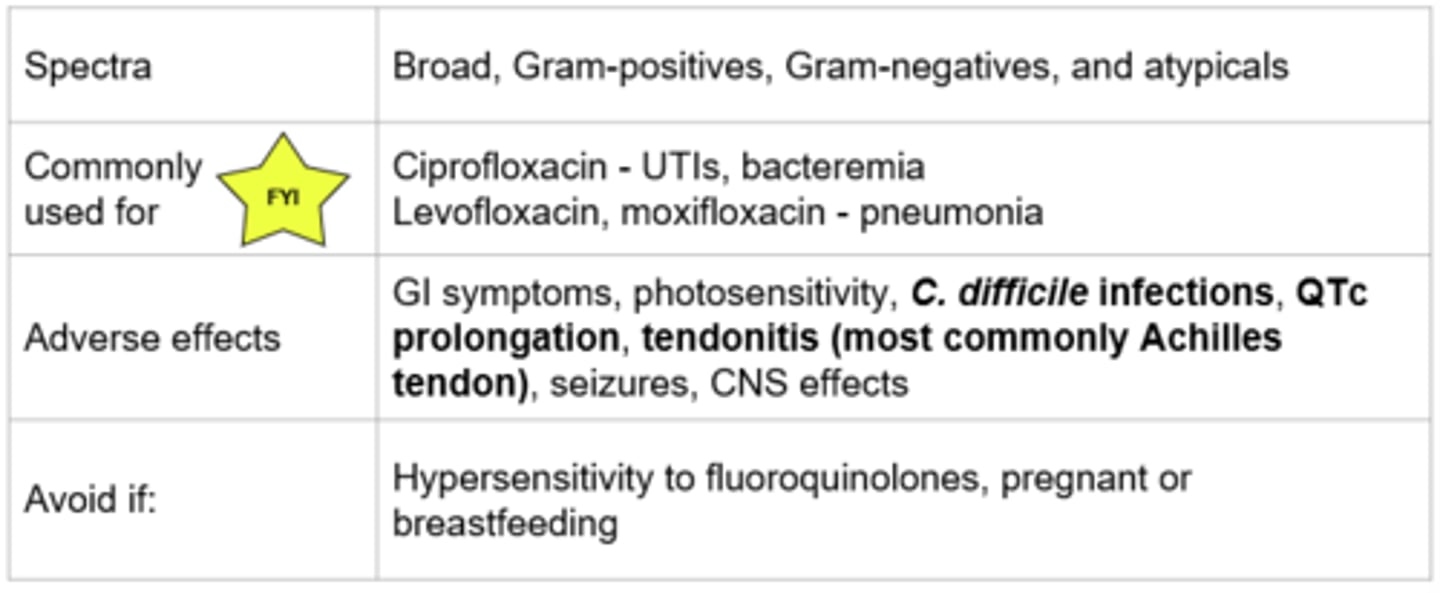
Fluroquinolones & Metal Cations
- Fluoroquinolones bind bivalent or trivalent metal cations → Decrease absorption
Avoid administering oral fluoroquinolones with bivalent or trivalent metal cations by 2 hours:
- This includes supplements such as: zinc, magnesium, iron, calcium (ex. TUMS and other antacids, multivitamins, iron supplements)
Metronidazole - Mechanism of Action
- Becomes reduced by anaerobic organisms → becomes cytotoxic free radical that breaks DNA, inhibits nucleic acid synthesis and results in loss of DNA integrity → cell death
- Bactericidal, concentration dependent killing
*Free radical formation
Metronidazole
Alcohol Reaction Signs = Nausea, vomiting, flushing, and hypotension

Sulfamethoxazole/Trimethoprim - Mechanism of Action
- Sulfamethoxazole (SMX) and trimethoprim (TMP) work together synergistically
- Inhibits folic acid synthesis which is necessary for DNA synthesis
- Bactericidal; time dependent killing
*Folic acid synthesis
Sulfamethoxazole/Trimethoprim

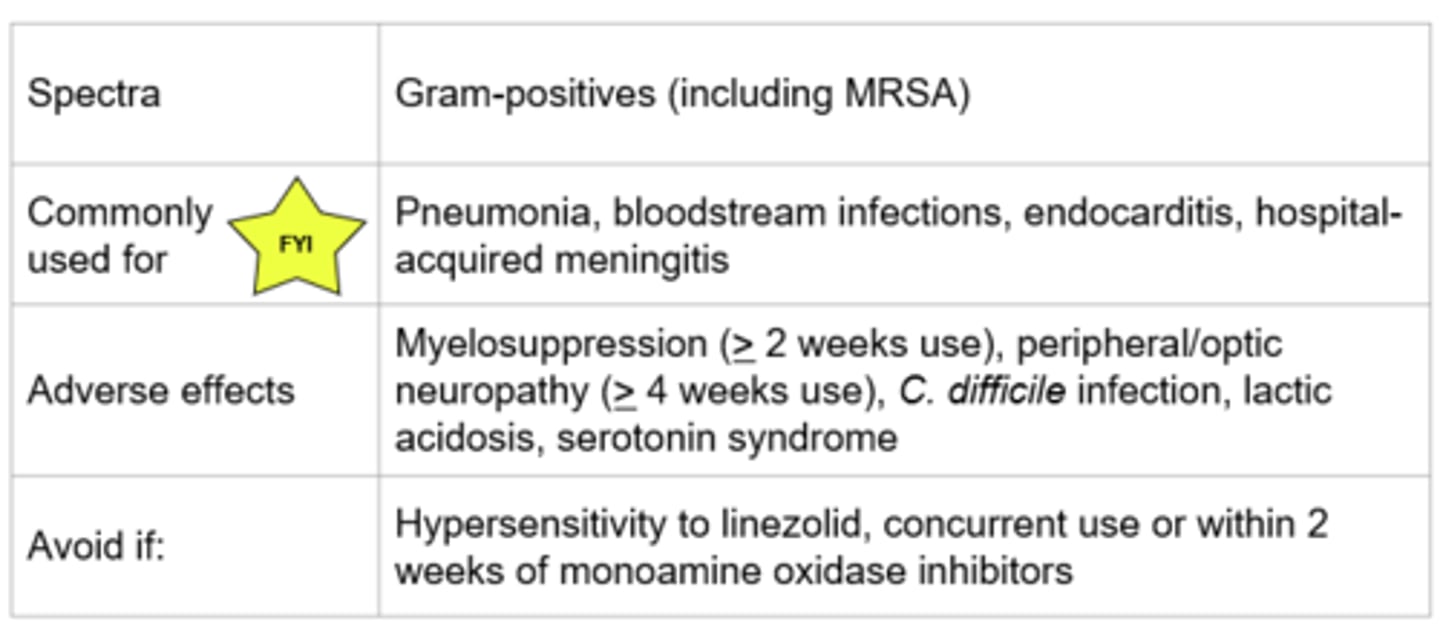
Linezolid - Mechanism of Action
- Binds to the P-site of the 50S ribosomal unit → prevents formation of the 70S complex
- Bactericidal against streptococci and bacteriostatic against staphylococci and enterococci
- Time dependent killing
*Protein synthesis
Linezolid
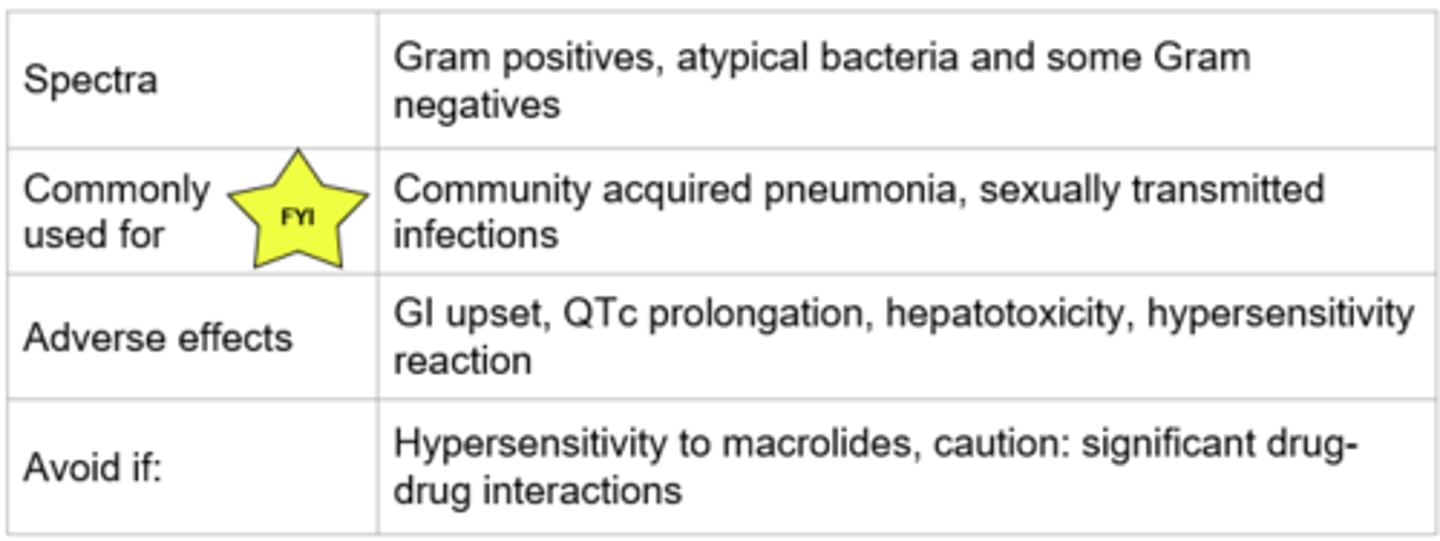
Macrolides - Mechanism of Action
- Reversibly binds to the 50S ribosomal subunit → prevents transpeptidation → protein synthesis inhibited
- Bacteriostatic, time dependent killing
- Examples (*ACE): Azithromycin, clarithromycin, erythromycin
*Protein synthesis
Macrolides
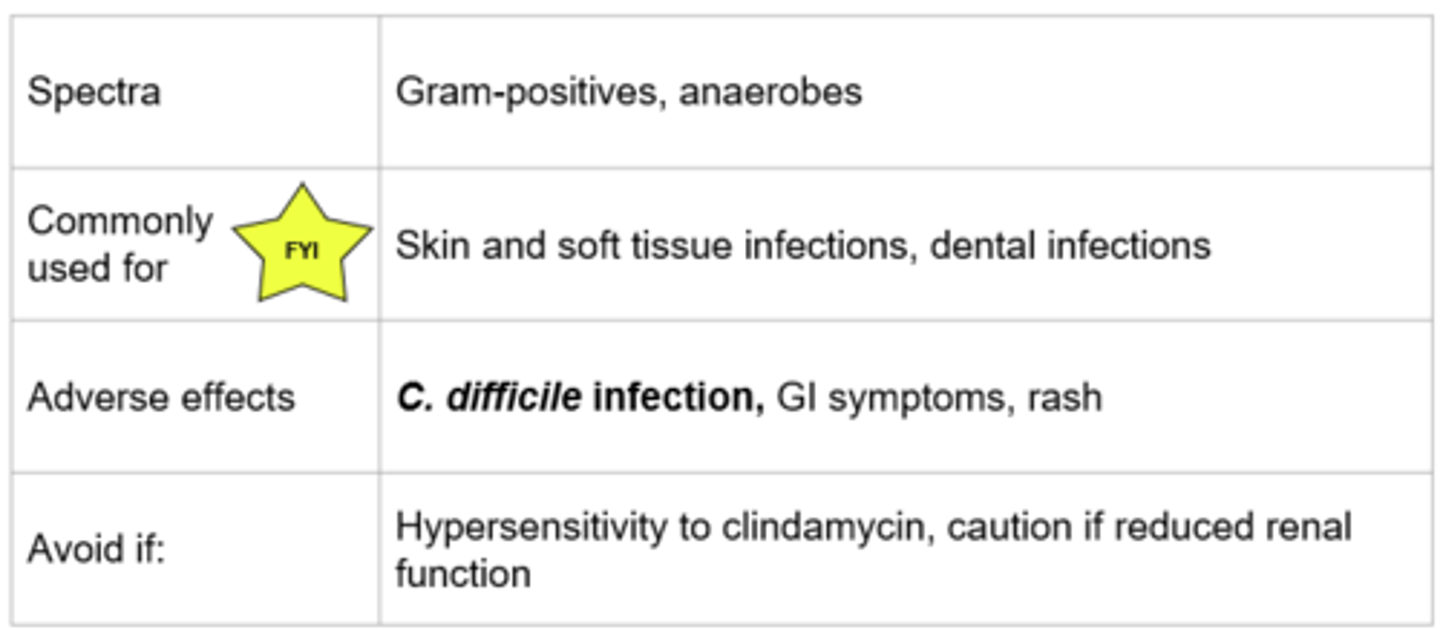
Clindamycin - Mechanism of Action
- Similar mechanism to macrolides: reversibly binds to 50S ribosomal subunit → inhibits transpeptidation → protein synthesis inhibited
- Bacteriostatic, time dependent killing
*Protein synthesis
Clindamycin
**MOST MAJOR FACTOR FOR DEVELOPING C DIFF!!*
Aminoglycosides - Mechanism of Action
- Binds irreversibly to the 30S ribosomal subunit → prevents transpeptidation → protein synthesis inhibited
- Bactericidal, concentration dependent killing
- Examples (*TAG): Gentamicin, tobramycin, amikacin
*Protein synthesis
Aminoglycosides
Vestibular toxicity - Damage to inner structures of ear (troubles with balance)
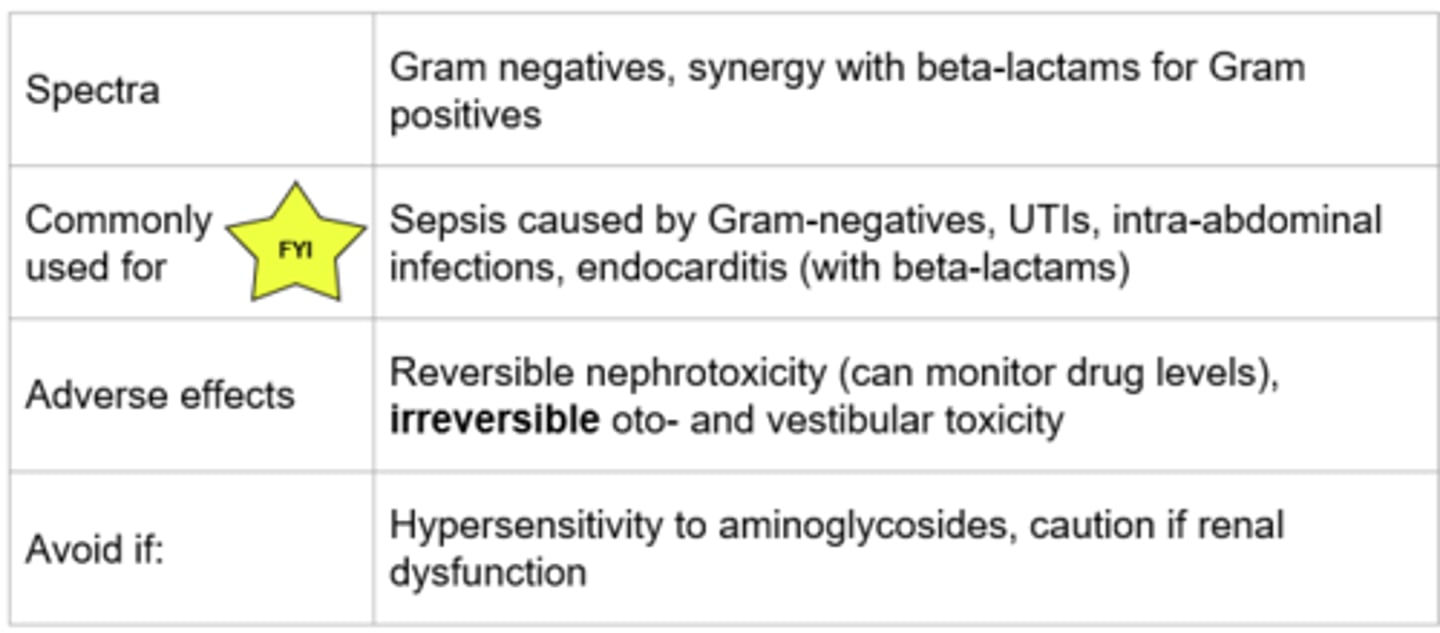
Aminoglycosides - Dosing & Monitoring
Extended-interval dosing: single, large dose once daily:
- More rapid bactericidal activity and less toxic
- Recommended for most infections in most patients
Traditional dosing:
- Smaller doses given multiple times a day
- Used when can’t use extended-interval dosing and for synergy
Therapeutic Drug Monitoring:
- Troughs are used to monitor for toxicity: drawn 30 minutes prior to next dose
- Peaks are used to measure efficacy: drawn 30 minutes after infusion ends
- Targets levels vary
Tetracyclines - Mechanism of Action
- Bind to the 30S ribosomal subunit → blocks the attachment of tRNA to mRNA-ribosome complex → protein synthesis inhibited
- Bacteriostatic, time dependent killing
- Examples: Tetracycline, doxycycline, minocycline
*Protein synthesis
Tetracyclines

Fungus
*Eukaryotic cells
Three types of Fungi:
Yeast
- Single celled, budding reproduction
- Ex. Candida species
Mold
- Multi cellular, branching filaments
- Ex. Aspergillus species
Dimorphic fungi
- Yeast at higher temps (37°C)
- Mold at lower temps (25°C)
- Ex. Histoplasma
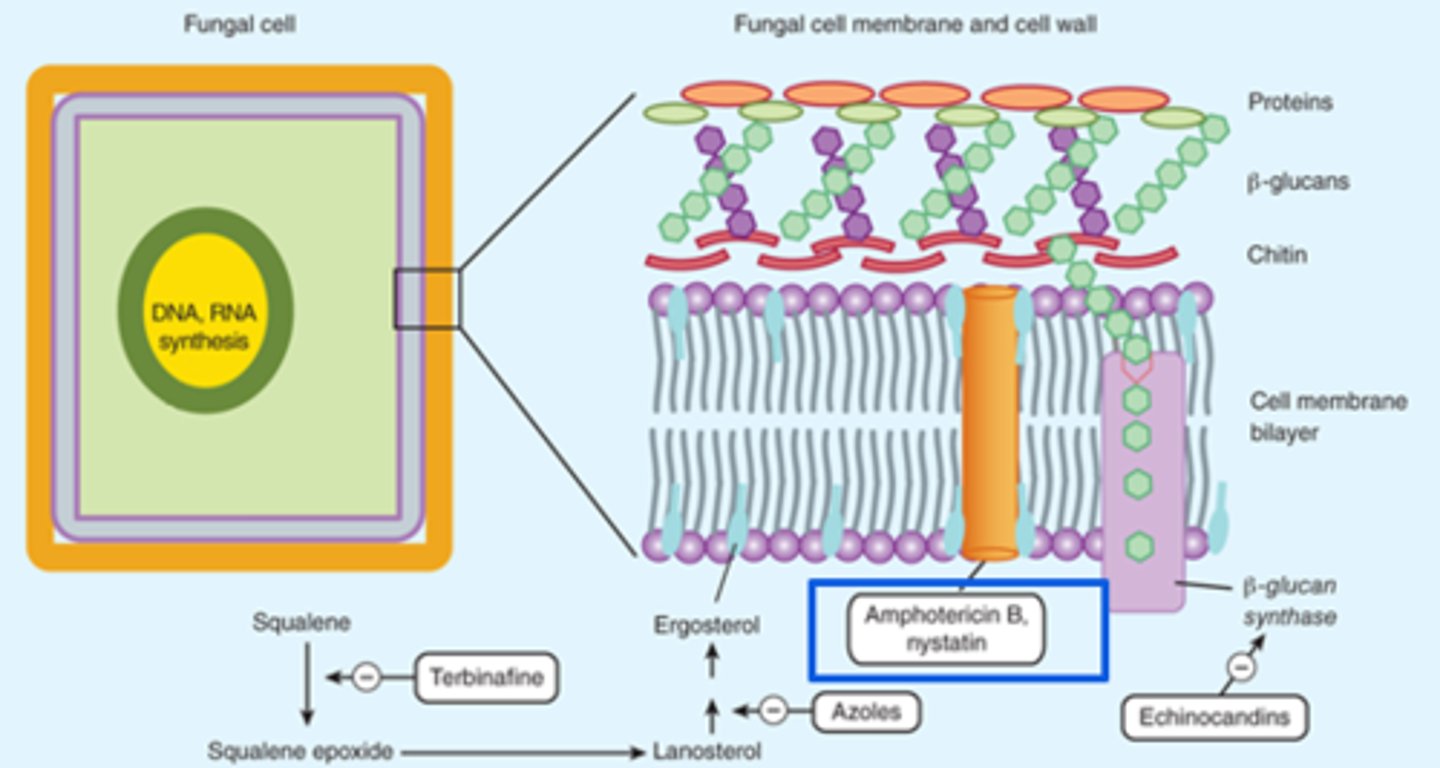
Fungal Cell Structure
Cell Membrane:
- Ergosterol: Azoles and terbinafine target ergosterol synthesis
Cell Wall:
- Chitin and Beta-glucan
- Polyoxins inhibit chitin synthase
- Echinocandins inhibit Beta-glucans synthesis
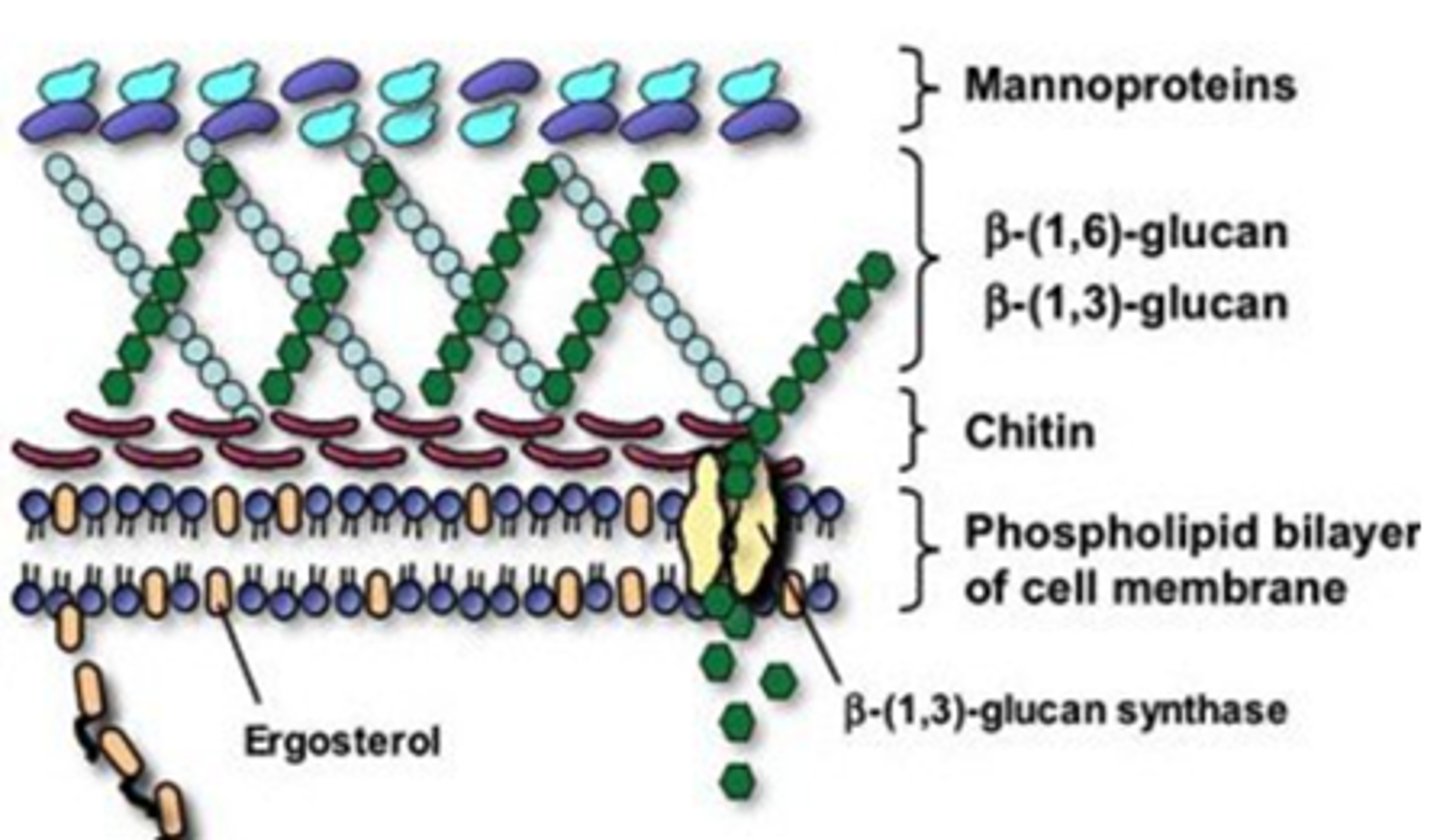
Antifungal Targets
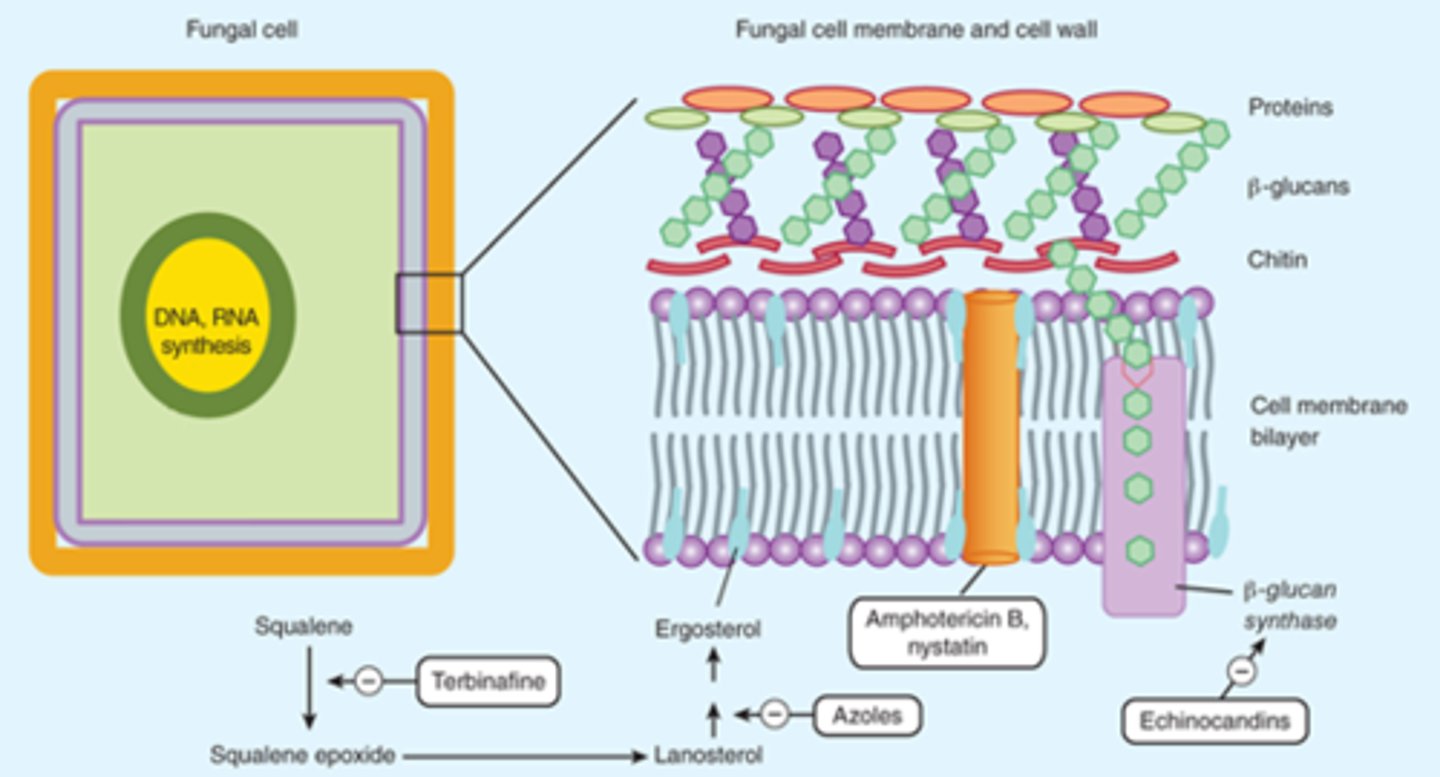
Polyenes
*Cell Wall Disruption
Polyenes - Mechanism of Action
- Binds to ergosterol in the fungal membrane → form a pore through the membrane → electrolyte leakage → cell death
- Resistance: Rare, due to decrease or change in structure of ergosterol
- Fungicidal
*Cell Wall Disruption
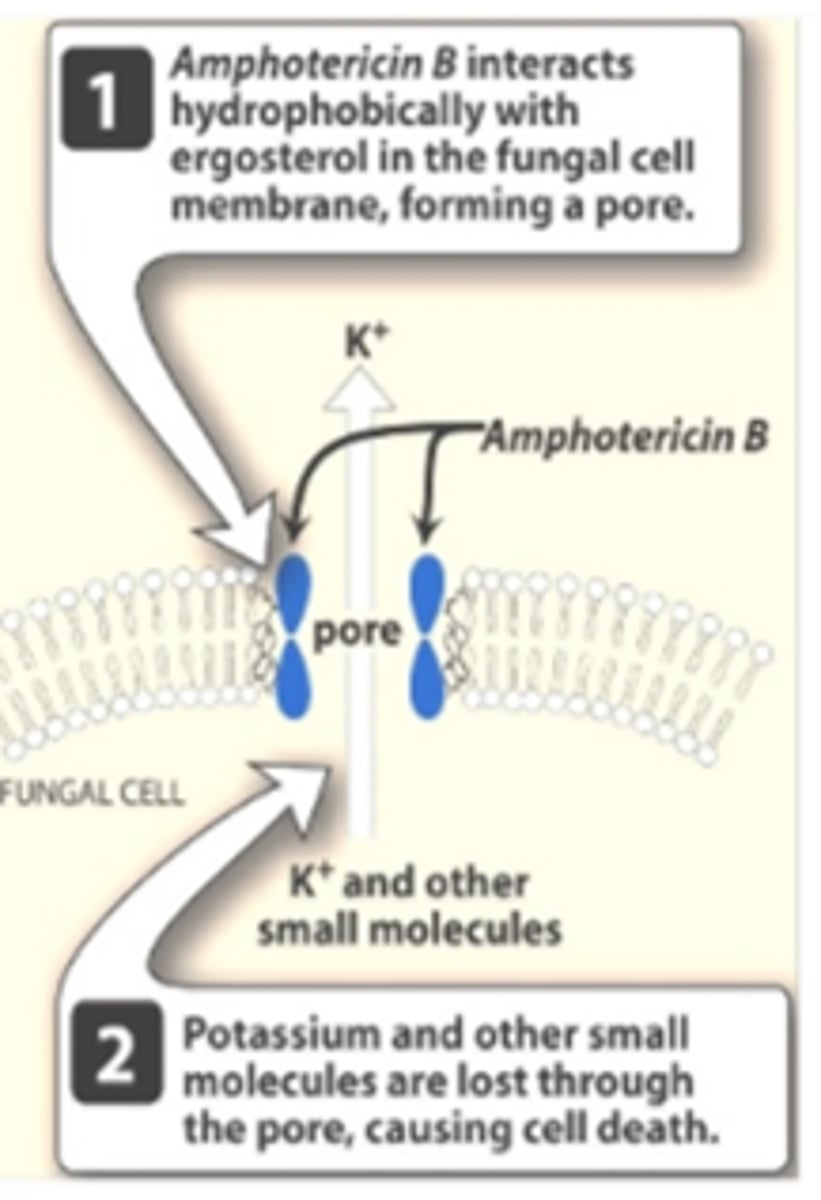
Polyenes - Nystatin
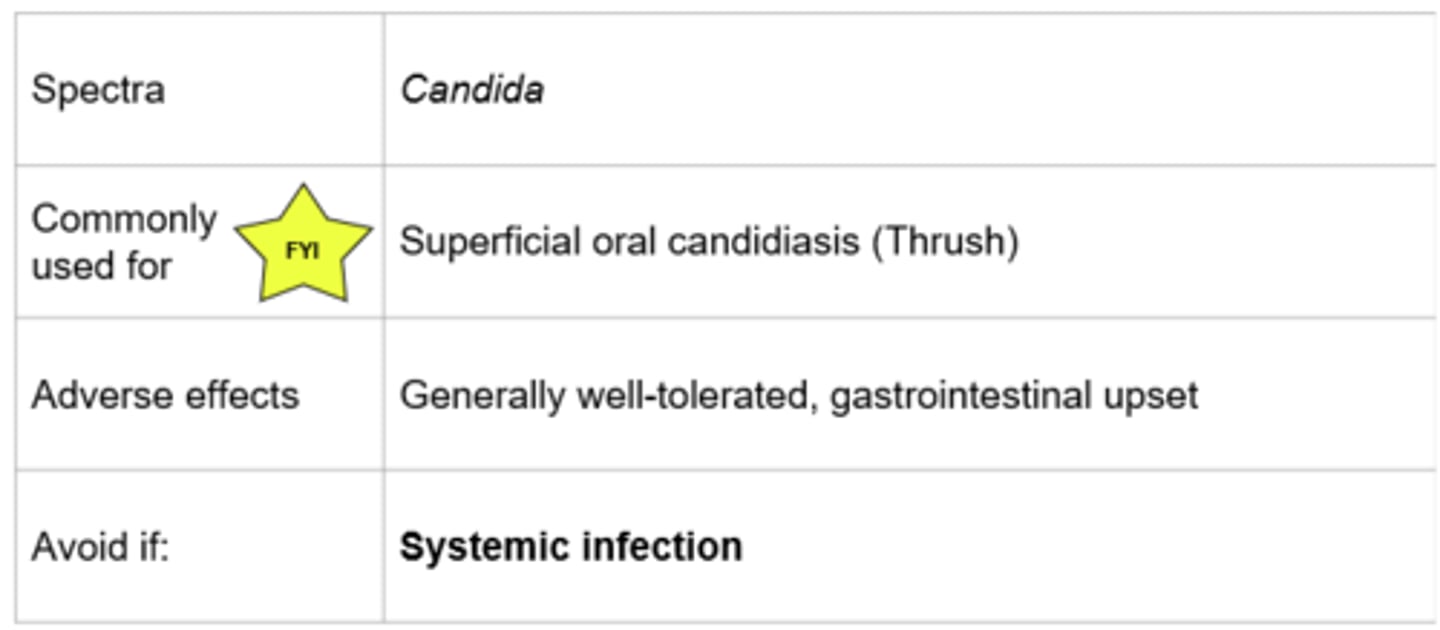
Nystatin
- Oral suspension: Swish and swallow/spit
- Poorly absorbed, cannot be used for systemic infections
Polyenes - Amphotericin B
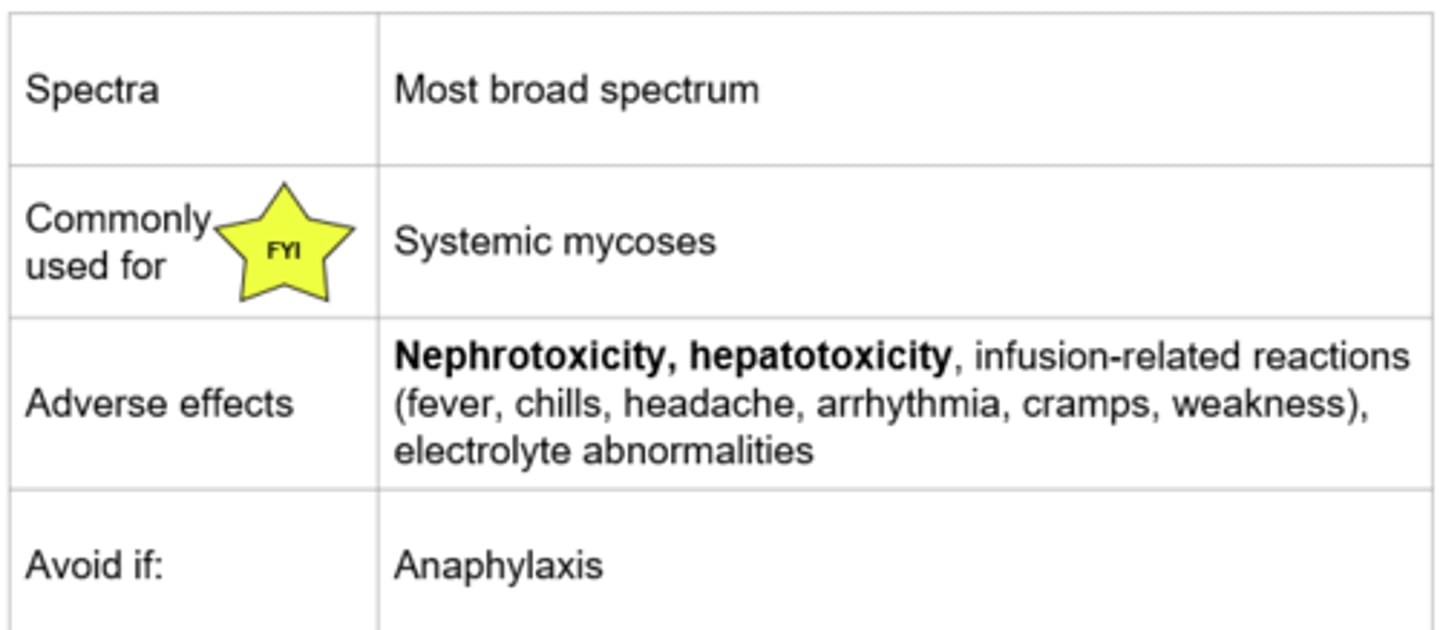
Amphotericin B
Lipid formulation available (drug enclosed in fat capsule to lower effects on renal system; more tolerable during infusion):
- Less nephrotoxic and fewer infusion related reactions
- Can reduce adverse effects by pre-treating with medications, prolonging the infusion, and providing patient with adequate hydration
- Pre-treatment includes: Fluids, antipyretics, antihistamines
Echinocandins
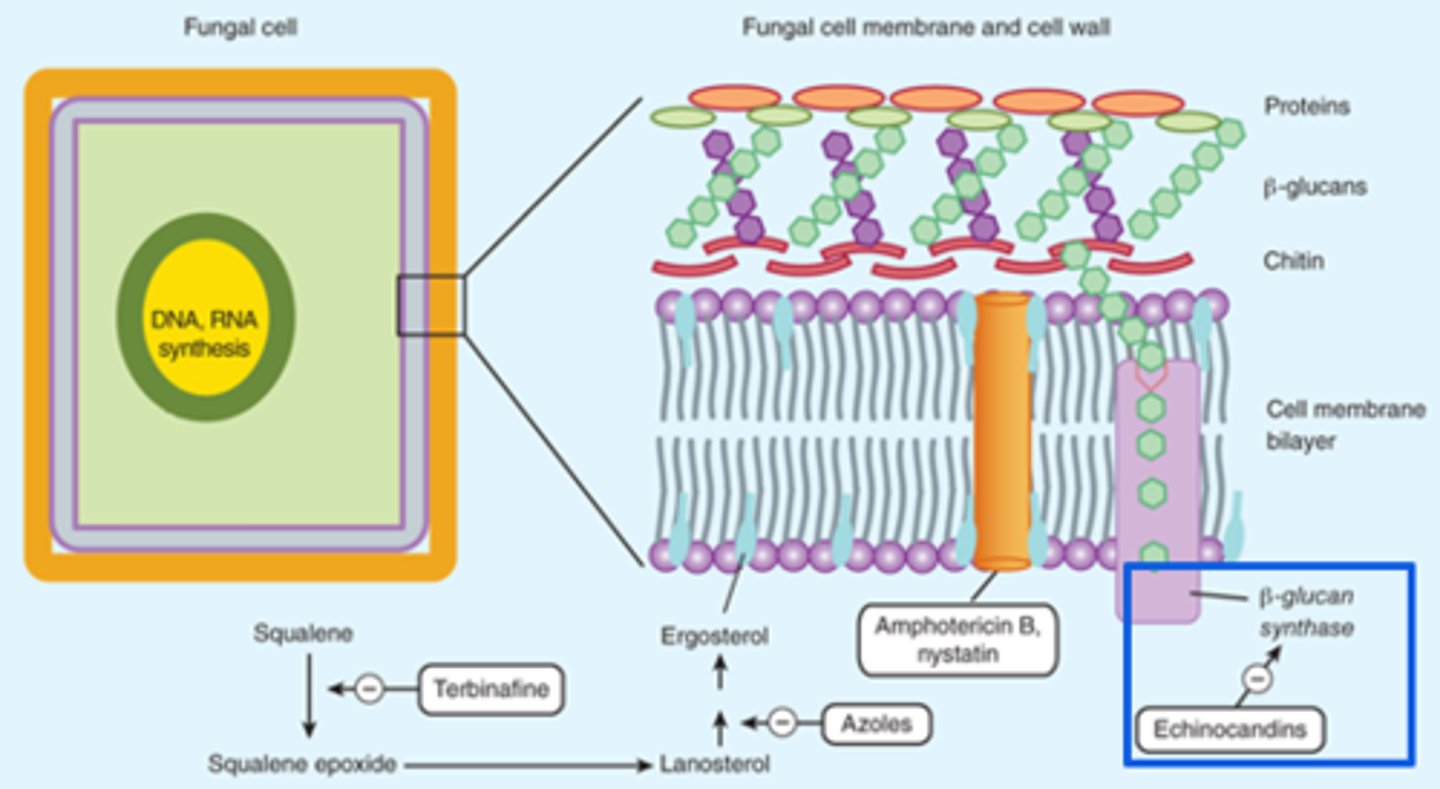
Echinocandins - Mechanism of Action
- Inhibits the synthesis of Beta (1,3)-D-glucan (component of the cell wall) by inhibiting Beta (1,3)-D-glucan synthase
- Impaired cell wall synthesis→ cell rupture and death
- Fungicidal (leads to cell rupture and death)(Candida - yeast) or Fungistatic (inhibits growth) (Aspergillus - mold)
*Cell wall synthesis
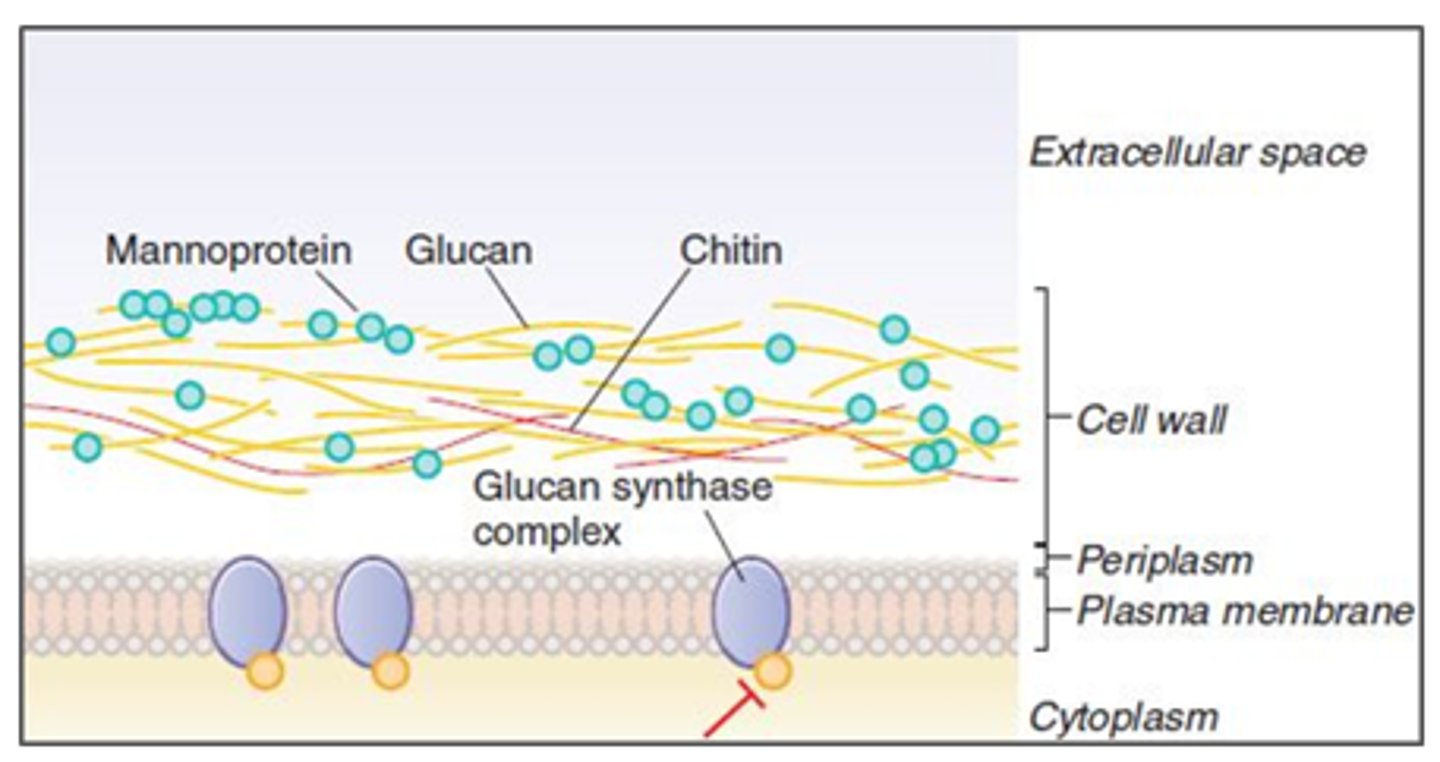
Echinocandins - Caspofungin, Anidulafungin, Micafungin
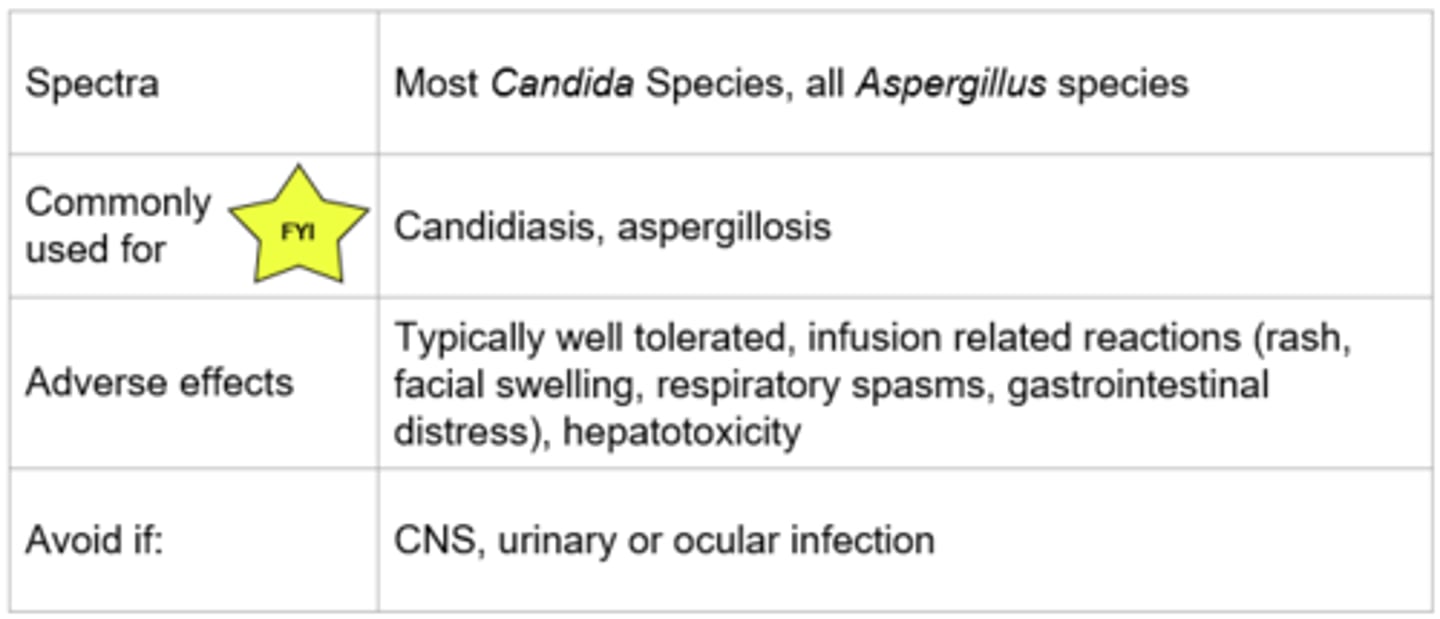
Echinocandins - Monitoring
- Monitor for potential infusion reactions (ex. facial swelling, flushing, rash)
- Monitor liver function (caspofungin requires dose adjustment in hepatic impairment)
- IV ONLY; if oral therapy is clinically warranted and the infection is susceptible, a switch to an alternate antifungal would be required
- Poor penetration to CNS, vitreal fluid and urine
- Very few drug interactions
Azoles
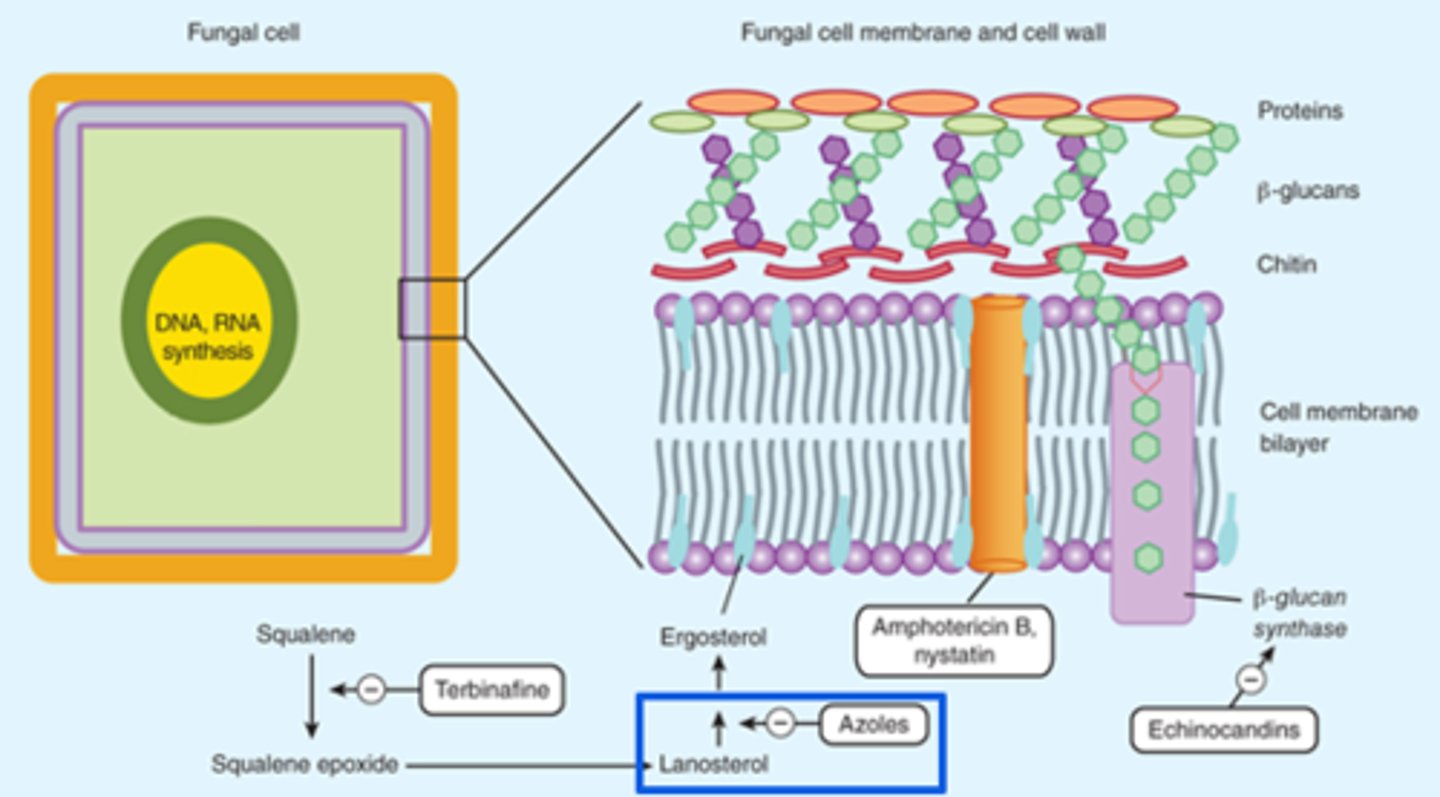
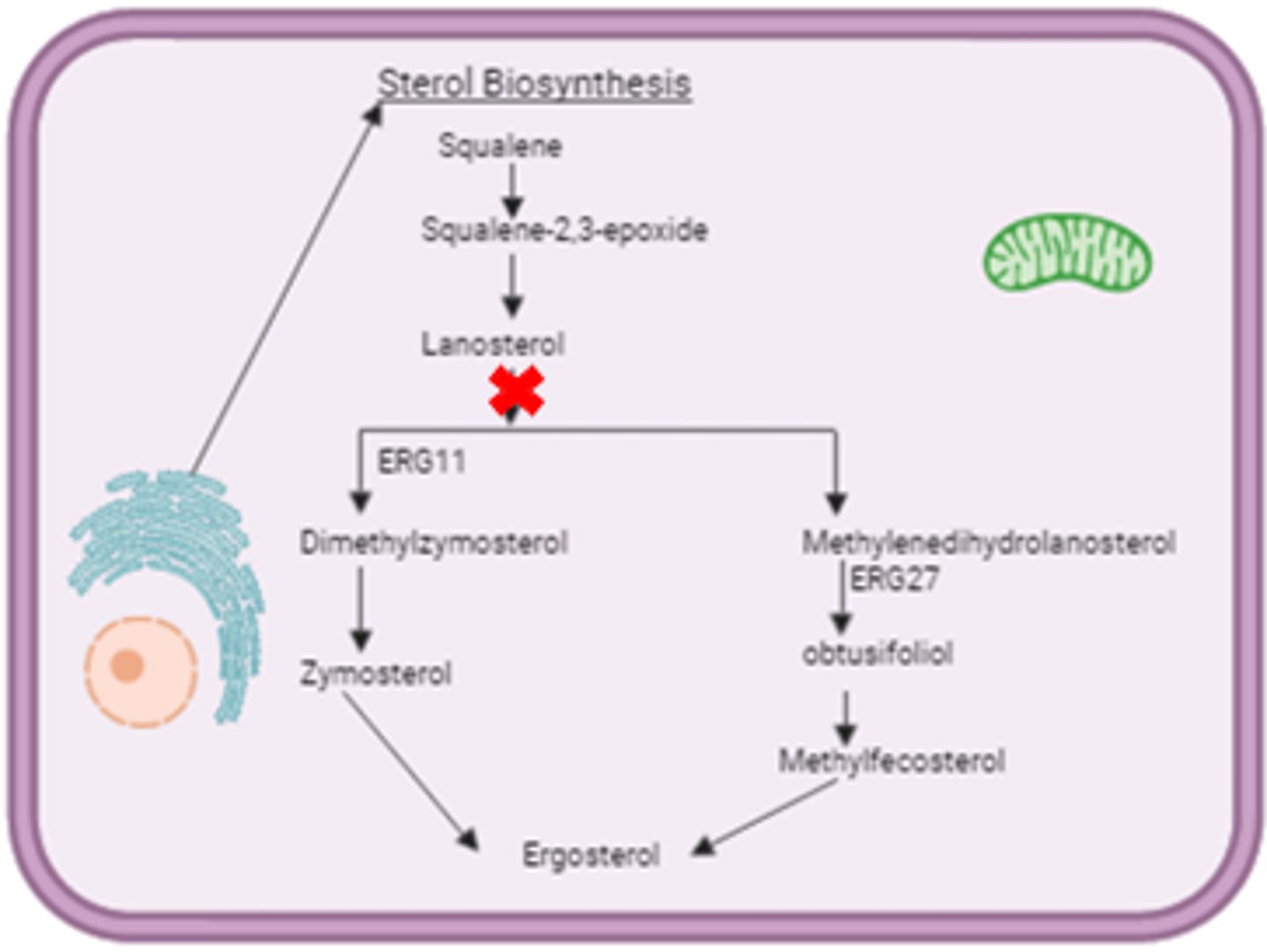
Azoles - Mechanism of Action
- Inhibits cytochrome P450 14-alpha lanosterol demethylase → inhibits conversion of lanosterol to ergosterol → increases permeability and accumulation of toxic sterols → cell death
- Resistance: Altered drug binding site
- Fungicidal or fungistatic (depending on species)
*Cell wall synthesis
Azoles - Agents

Azoles

Azoles - Clinical Monitoring
- Intravenous to Oral Step Down: Bioavailability is typically high and oral may be used even for deep seated infections
- Many drug interactions!
- Renal and hepatic function must be monitored on oral/IV therapy; QTc prolongation can occur with any systemic azole
- If susceptible, use fluconazole as it has the best safety profile + excellent oral bioavailability + excellent penetration into CNS/vitreal fluid
Terbinafine
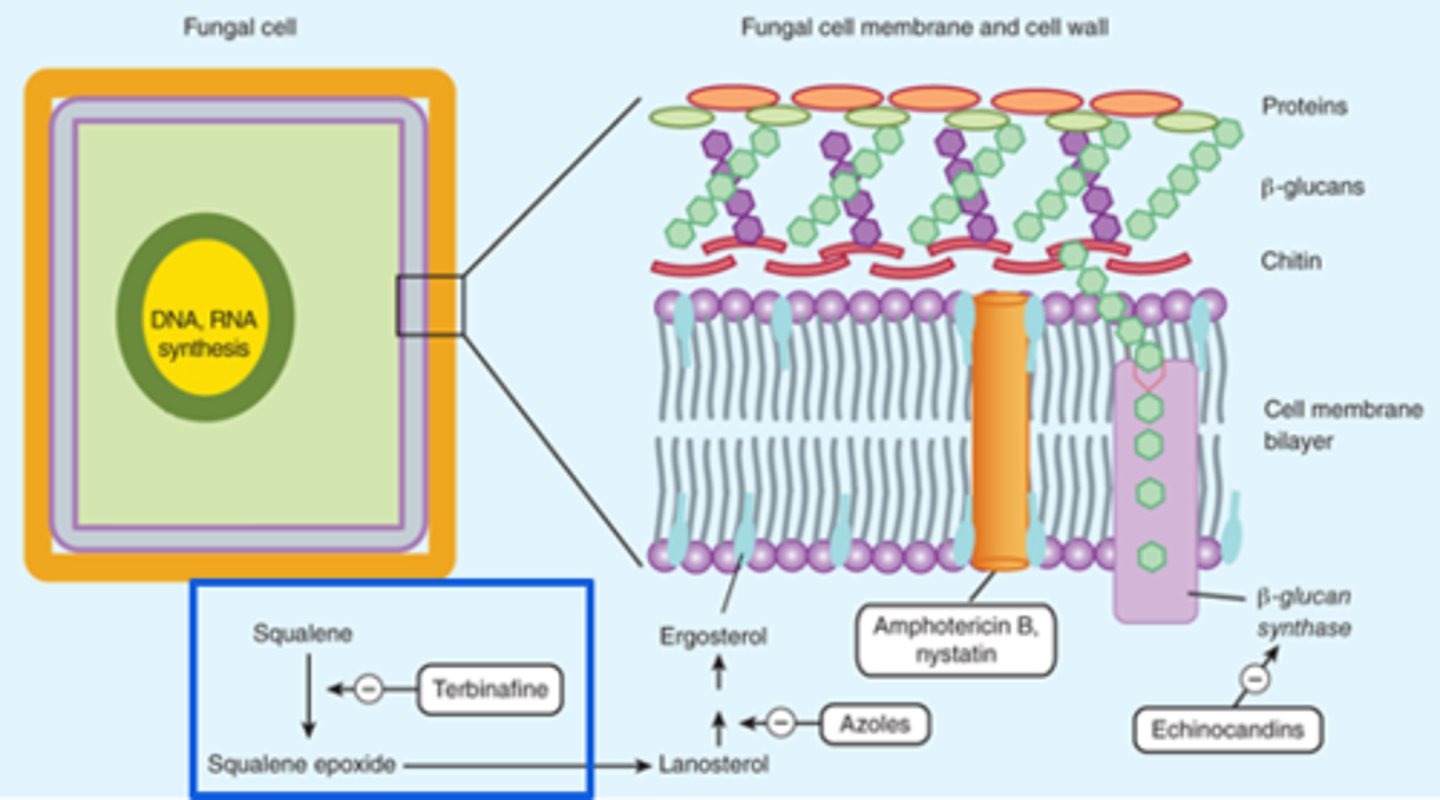
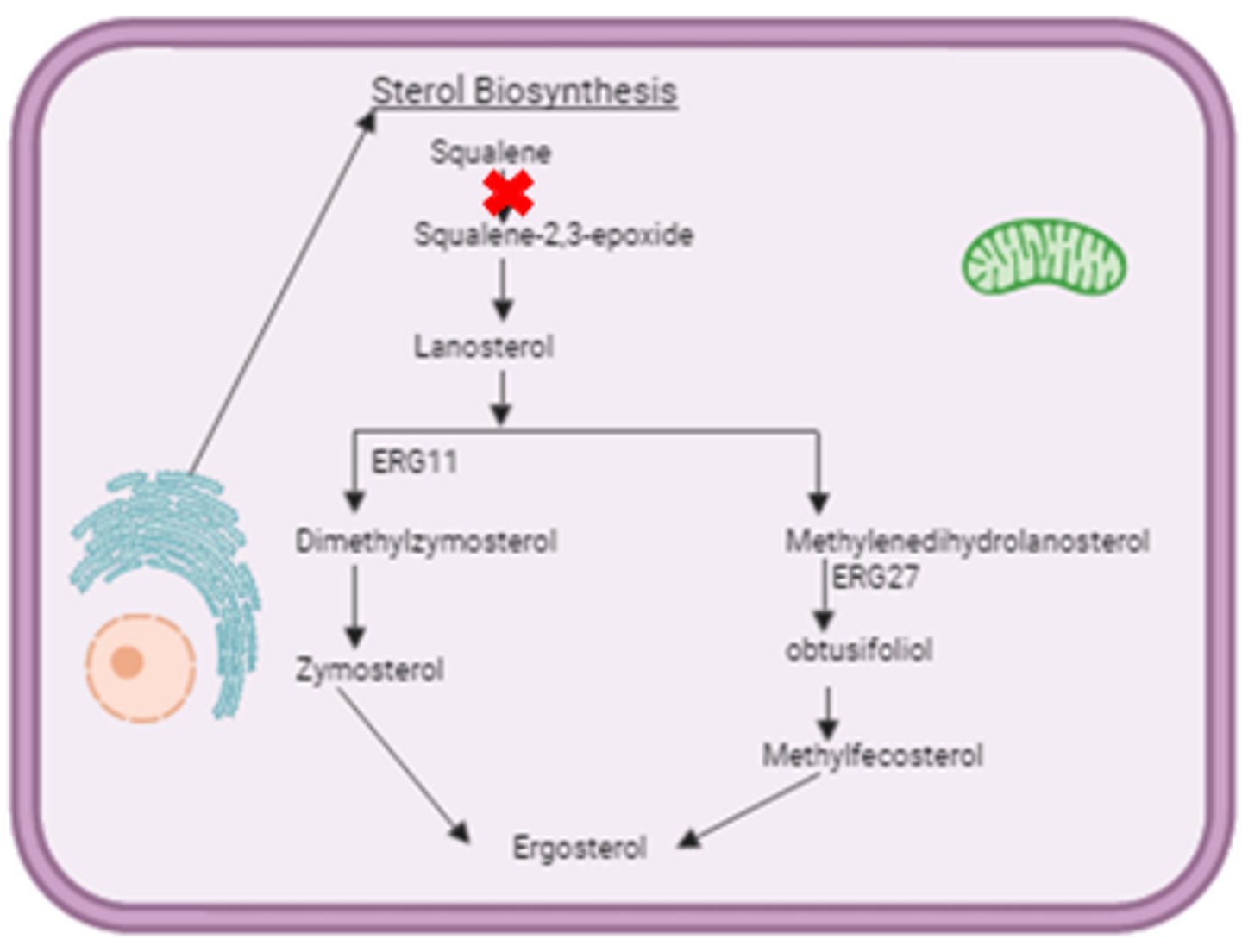
Terbinafine - Mechanisms of Action
- Interferes with squalene epoxidase → inhibit ergosterol biosynthesis → alter cell membrane permeability and accumulation of toxic sterols → cell death
- Resistance: mutation in squalene epoxidase
- Fungicidal
*Cell wall synthesis
Terbinafine
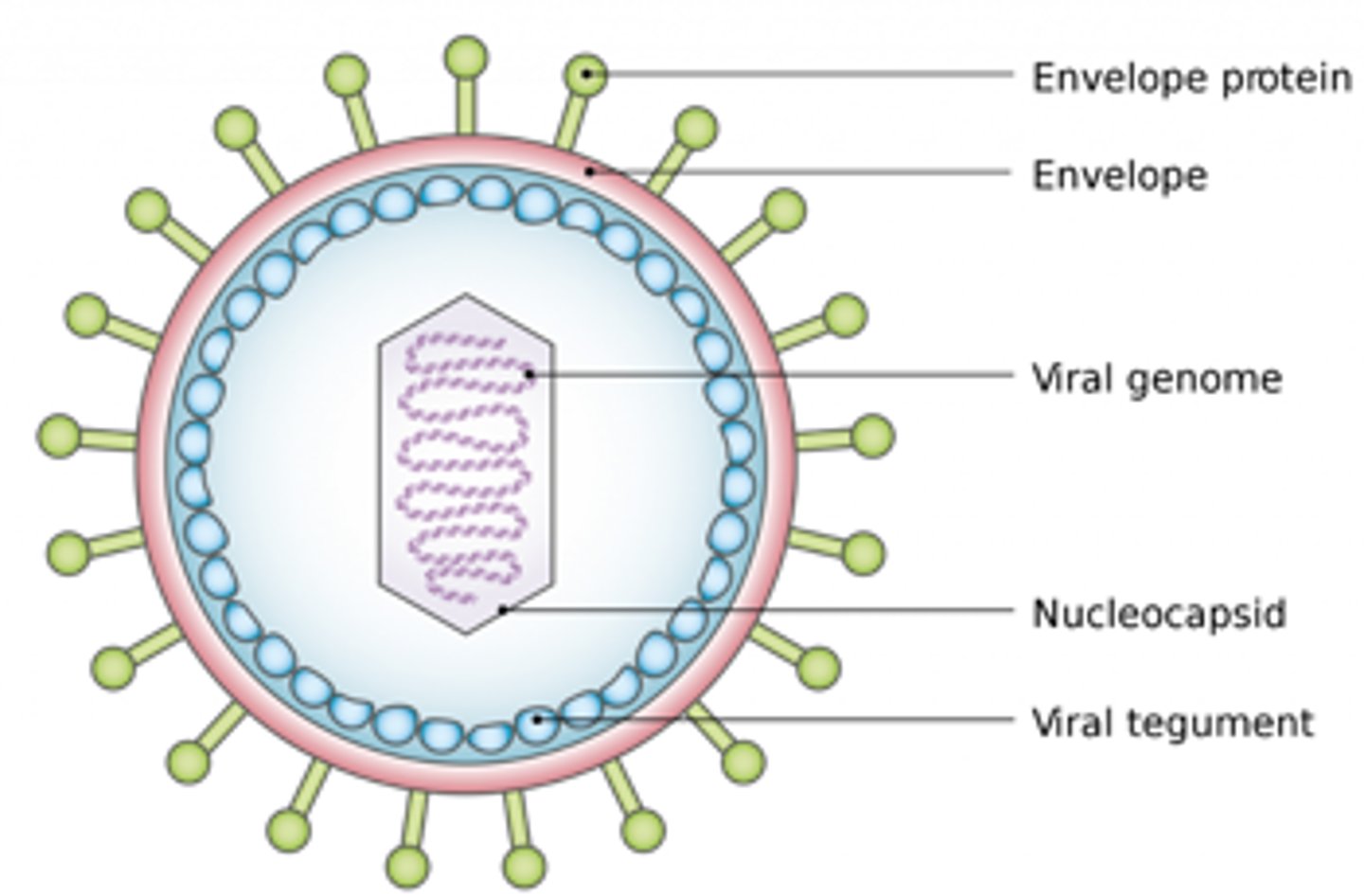
Viruses
- Consist of DNA or RNA within a protein outer shell
- Require the use of host cell machinery to replicate; cannot replicate on their own
- Infect host cells and "re-program" to replicate and the virus
Viral Infections
Types of Antivirals
- Antiviral agents
- Neuraminidase inhibitors
- Polymerase inhibitors
Neuraminidase Inhibitors - Mechanism of Action
*ONLY FOR THE FLU!!
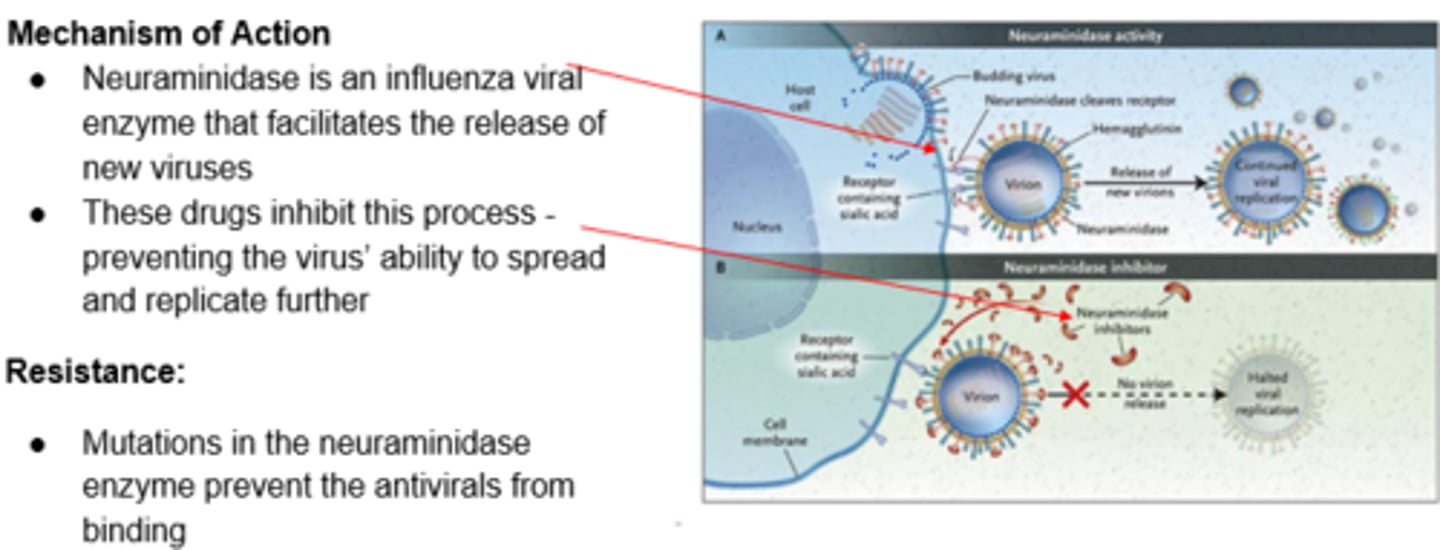
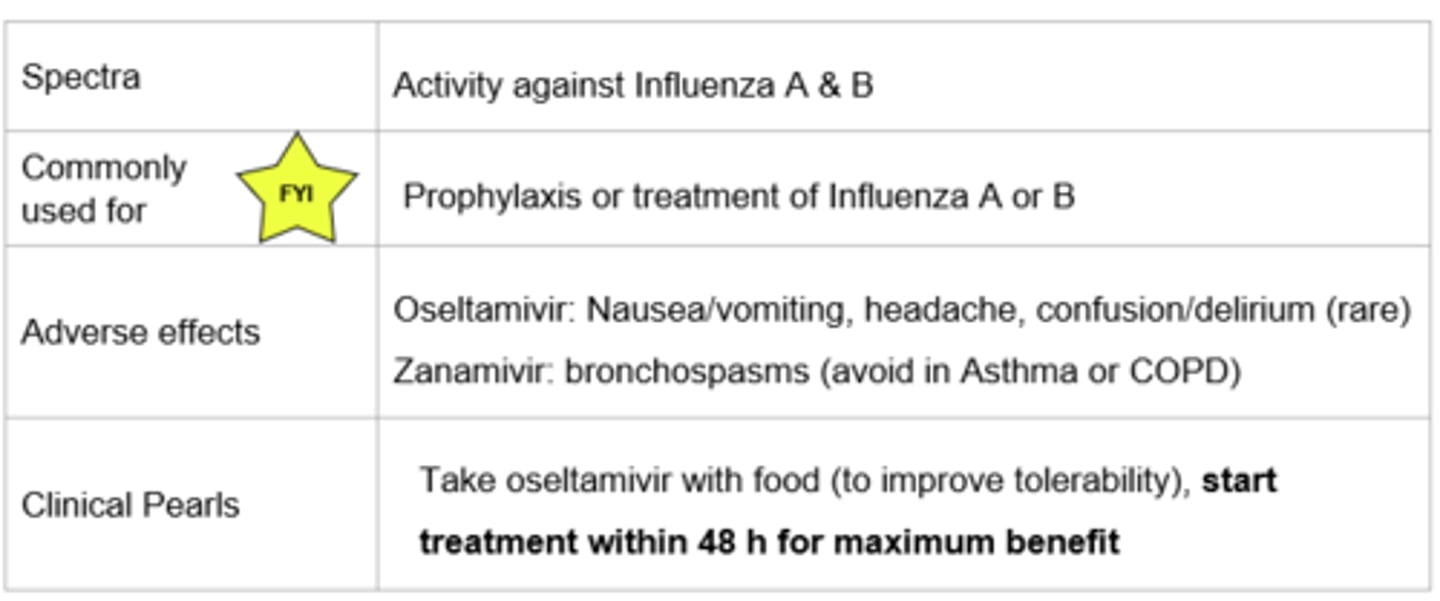
Neuraminidase Inhibitors - Oseltamivir (PO), Zanamivir (Inhaled)
Polymerase Inhibitors - Mechanism of Action
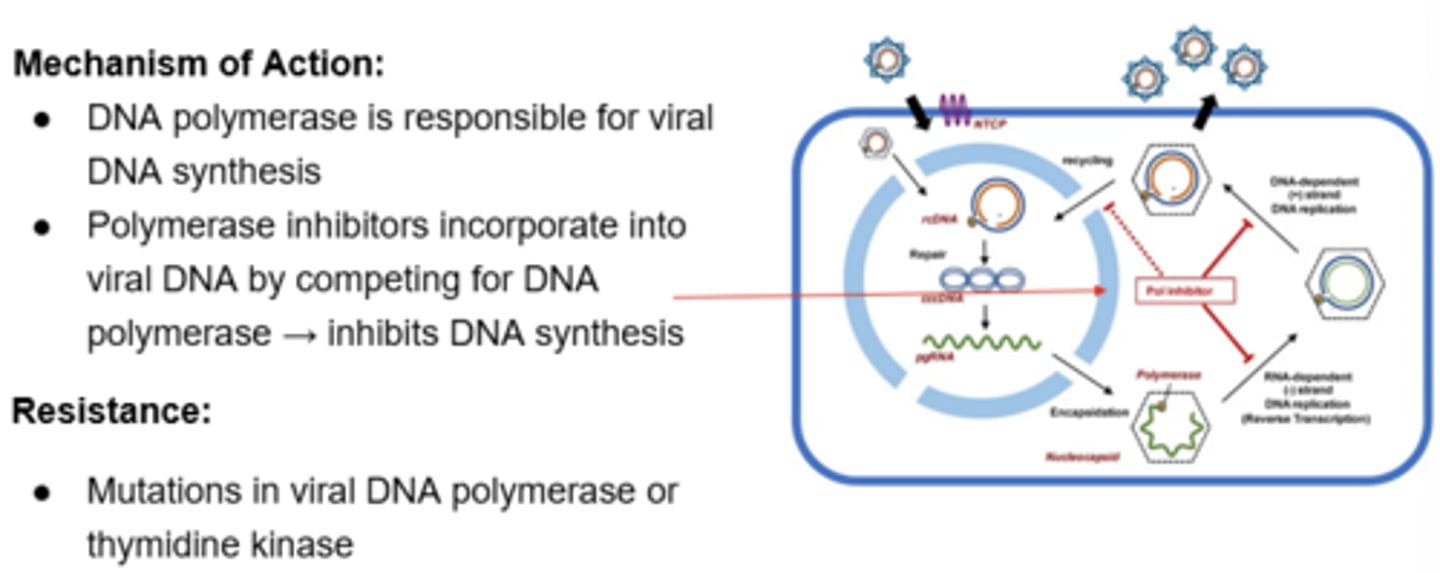
Polymerase Inhibitors - Narrow Spectrum: Acyclovir (IV/PO), Valacyclovir (PO), Famciclovir (PO)

Polymerase Inhibitors - Broad Spectrum: Ganciclovir (IV/PO), Valganciclovir (PO)

Patient KA is receiving vancomycin for management of cystic fibrosis. A physician orders a vancomycin trough level. When should the blood be drawn to obtain an accurate trough level?
a) Immediately after the entire dose of vancomycin is administered
b) During the vancomycin infusion
c) Two hours prior to the next vancomycin dose
d) Thirty minutes prior to the next vancomycin dose
Answer = c
NS presents to the ER again with pneumonia. The physician decides to start her on ciprofloxacin 750 mg by mouth every 12 hours for 7 days.
What are some side effects to monitor for?
a) Kidney dysfunction
b) Hyperkalemia
c) Infusion reaction
d) Tendinopathy
Answer = d
Doxycycline should be avoided as a treatment in which patient?
a) A 2-year old male with an ear infection, but is otherwise healthy
b) A 15 year old female with acne
c) A 45 year old male with an allergy to amoxicillin
d) A 87 year old female with liver failure
Answer = a
Patient AF is being treated for histoplasmosis with amphotericin B. Which of the following side effects are most likely to occur?
a) Diarrhea
b) Bradycardia
c) Hypertension
d) Fever/chills
Answer = d
Patient AJ is being treated for HSV encephalitis with IV Acyclovir. Which of the following statements is correct?
a) AJ should take acyclovir on an empty stomach to enhance the drugs absorption in the GI tract
b) AJ requires proper hydration to prevent nephrotoxicity by reducing the risk of crystallization int he kidneys
c) IV acyclovir should be infused slowly to increase its effectiveness against viral infections
d) IV acyclovir should be infused quickly to speed up its metabolism in the liver
Answer = b