Science Test 3 Y10T2 - Chemistry
1/25
Earn XP
Description and Tags
Quick and rushed knowt for the exam :(
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
The Periodic Law
The law created by Dmitri Mendeleev, in which he states that by arranging elements in order of their atomic number, patterns in their properties are formed. Through this, he arranged the periodic table in groups and properties to show the patterns (like valence electrons, and outer shells)
Groups in the Periodic Table
The vertical columns which are number 1-18, and groups elements that have the same charge when gaining stability (e.g., potassium and sodium both get a charge of +1 as they are in the same column), and have similar properties
Periods in the Periodic Table
The horizontal rows which are numbered from 1-7 which group elements with the same amount of outer shells. In the first row, there are only 2 elements, due to the first outer shell only being to contain two electrons
Alkali Metals
Most of Group 1, and is lithium , sodium, potassium, rubidium, caesium, and francium. One of the most reactive groups, forming a cation, and has a charge of 1+ when stable. Are soft, low density, low in melting/boiling points, and become more reactive as you go down, as the more outer shells an atom has, the less attraction is has toward the valence electrons (as they are far away), allowing it to lose the electrons easier
Alkaline Earth Metals
Is Group 2, being beryllium, magnesium, calcium, strontium, barium, and radium. One of the second most reactive groups, forming cations with a charge of 2+. Are soft, low density, low in melting/boiling points, and become more reactive as you go down, as the more outer shells an atom has, the less attraction is has toward the valence electrons (as they are far away), allowing it to lose the electrons easier
Transition Metals
The elements between group 3 to 12, being able to form cations, and are fairly unreactive when compared to alkali metals and halogen, though can react. Are strong and hard, high density, and have high melting/boiling points
Halogens
The elements in the 17th group of the periodic table, including Fluorine, Chlorine, Bromine, Iodine, Astatine, and Tennessine. Form anions with a charge of 1-. Are brittle when solid, have low melting/boiling points, and become less reactive as you go down the group, due to having less attraction to pull in electrons as there are more outer shells in the way
Metals
The elements that include the alkali metals, alkaline earth metals, transition metals, and post-transition metals, which form cations, and bond together to create metallic bonds
Cations
Positively charged ions (ions with less electrons than protons)
Anions
Negatively charged ions (ions with more electrons than protons)
Metalloids
The group of Boron, Silicon, Germanium, Arsenic, Antimony, Tallurium, and Polerium which share properties with metals and non-metals, such as being as able to be anions or cations
Noble Gases
The elements of Group 18 which are completely unreactive due to having a full valence shell.
Non-metals
Elements that include the halogens, noble gases, and other non-metals (carbon, nitrogen, hydrogen, oxygen, phosphorous, sulfur, selenium), which if in ions, form anions, and when bonded together, create covalent bonds
Electron Shell Filling Order
2, 8, 8, 2, though this ends at Calcium and changes to 2, 8, 18
Valence Electron Shell
Outermost electron shell, which either seeks to be removed (Cations), to be filled (anions), or nothing (noble gases)
Neutral Atom vs Charged Ion
Neutral refers to an atom without any charge, meaning it has an equal amount of protons to electrons, while charged ions have more or less electrons than protons
Charges of Groups 1, 2, 13, 15, 16, 17 when an ion
Group 1: 1+
Group 2: 2+
Group 13: 3+
Group 15: 3-
Group 16: 2-
Group 17: 1-
Bond Naming Process
Ionic bonds always have the metals first, and then the non-metals second. The name will have the metals, and then the non metals with the amount of the nonmetal + ide/ine/etc. (will be on valency chart). Metallic bonds can only be made of one element, so it is just that, and covalent bonds have the first as its element, and only having its number if there is more than one of it (e.g., dihydrogen monoxide in H2O)
Ionic Bonding
A form of bonding between two or more metal and non metal ions, in which the metals transfer their valence electrons to the non-metals, allowing both to become stable
The Lattice in Ionic Bonding
The structure of ionic bonding, which is made up of alternating positive and negative ions that are equally spaced apart, and have a strong electrostatic attraction keeping them in place
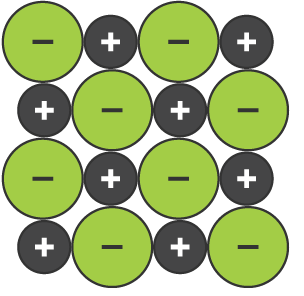
Properties of Ionic Bonding
Non-malleable due to movement the positive ions will put them closer other positive ions, and cause them to repel (same as with negative ions)
Non-conductive when solid as they are not moveable due to their strong electrostatic attraction
Conductive when liquid as they can move, and are charged particles
Have high melting/boiling points due to having strong electrostatic attractions (meaning much energy is required to break bonds)
Covalent Bonding
A form of bonding between two or more non-metals share one or more electrons with each other to allow both to reach stability
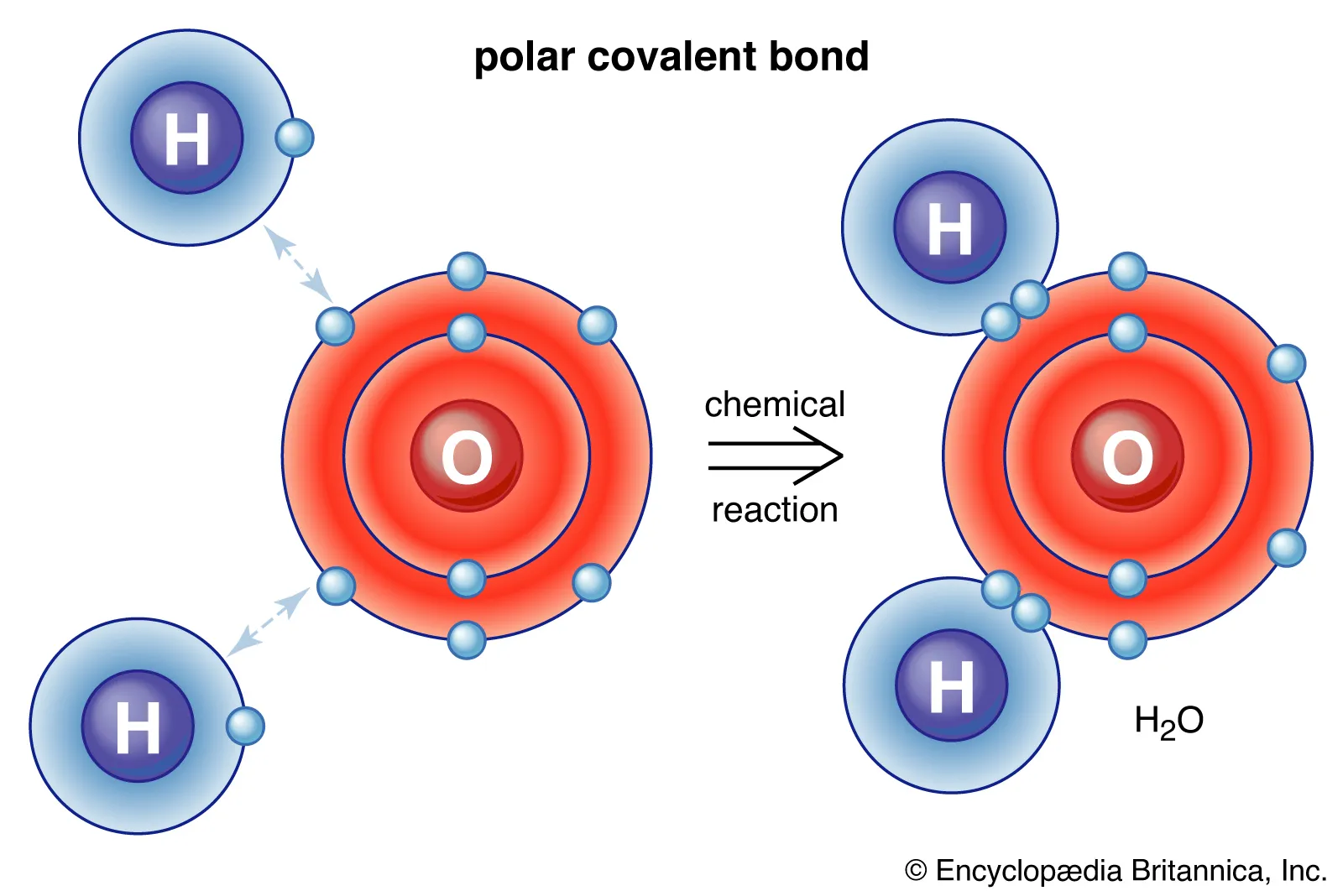
Covalent Bonding properties
Malleable as there is only little attraction (Van Der Wall’s charges) holding multiple molecules together, only having attraction to keep one molecule intact
Non-conductive as the molecule has a neutral charge due to there being no transfer of electrons, only sharing
Low melting/boiling points for the same reason as why they are malleable (there being little attraction connecting each molecule)
Metallic Bonding
A form of bonding between two or more of the same metal, in which the metals remove their extra valence electrons and form a layer of positive metal ions, connected by a sea of delocalised electrons, giving it a high electrostatic attraction keeping it in place
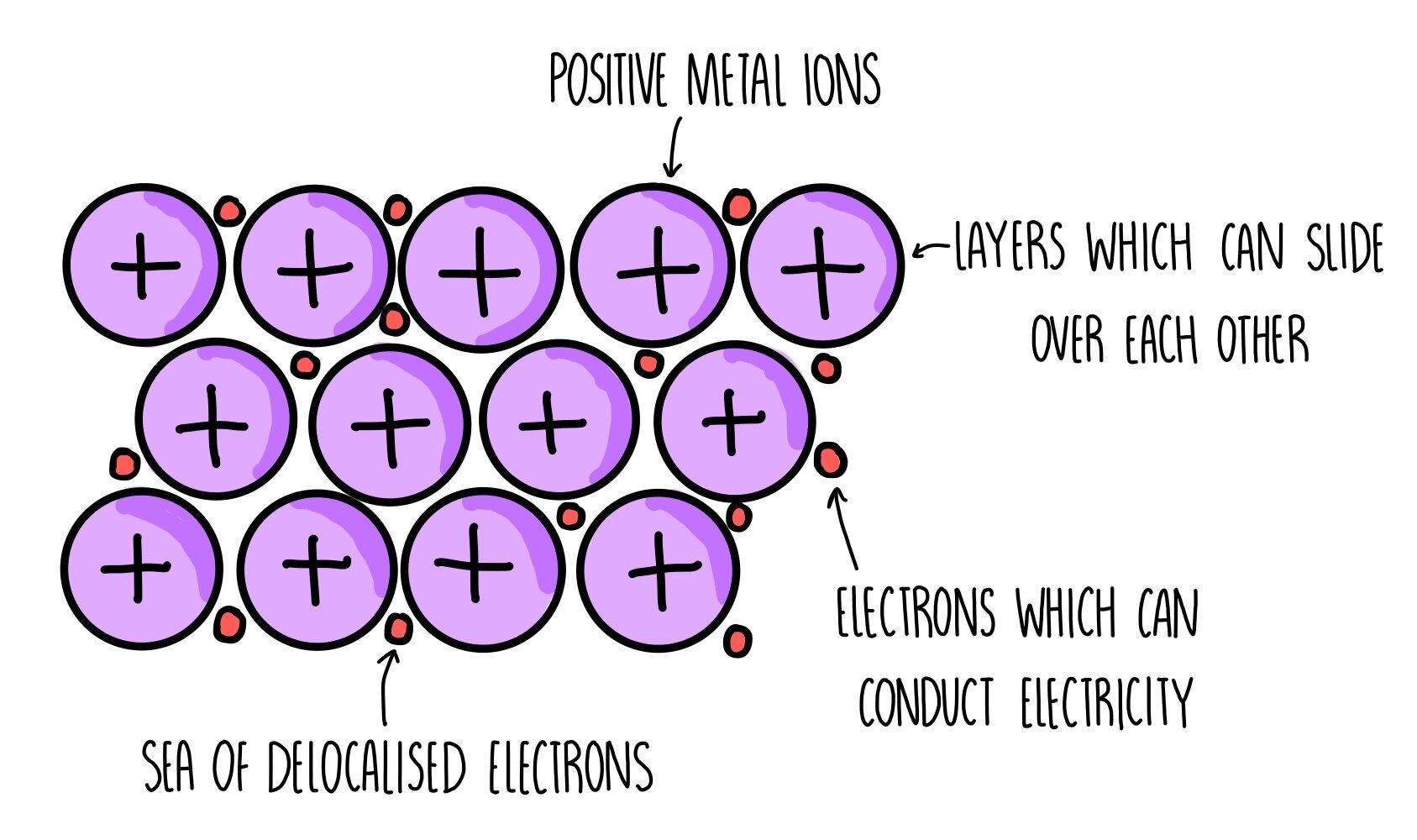
Metallic Bonding Properties
Malleable due to the sea of delocalised electrons still connecting metal ions when they move as the sea can move
Conductive due to the sea of delocalised electrons being able to carry charges
High melting and boiling points due to the strong attraction from the electrons keeping the positive metal ions together
Valency
How reactive a substance is