AP Bio Metabolism Test
5.0(2)
Card Sorting
1/63
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
1
New cards
metabolism
the totality of chemical reactions occurring within an organism; the metabolism of a cell manages the material & energy resources of the cell
2
New cards
anabolic pathway
invest energy to build large molecules from smaller molecules (Ex. Photosynthesis)
-gaining free energy because its becoming larger and less stable, surrounding area loses free energy for use and decreasing entropy
-gaining free energy because its becoming larger and less stable, surrounding area loses free energy for use and decreasing entropy
3
New cards
catabolic pathway
break large molecules down into smaller molecules and release energy. (Ex. Cellular respiration; glucose and other organic fuels are broken down in the presence of oxygen to carbon dioxide and water)
-so losing free energy because it's smaller molecules with more stability, but the surrounding area gains free energy which means more entropy
-so losing free energy because it's smaller molecules with more stability, but the surrounding area gains free energy which means more entropy
4
New cards
energy coupling
when the energy released from catabolic pathway can be stored and used to drive catabolic pathways
-can help metabolism from reaching equilibrium, although it is mostly because things are always moving in and out of cell and being recycled
-can help metabolism from reaching equilibrium, although it is mostly because things are always moving in and out of cell and being recycled
5
New cards
energy
capacity to cause change or do work; Ability to move and/or rearrange matter
6
New cards
kinetic energy
Energy of movement; Moving objects impart movement on other matter
7
New cards
Thermal energy
KE associated w/ random movement of atoms & molecules
8
New cards
Electromagnetic energy
KE associated w/ photons traveling from sun
9
New cards
potential energy
stored energy w/ the capacity to do work; energy resulting from position or structure
10
New cards
Chemical energy
potential energy that can be released by chemical reactions (energy from food released as it is broken down)
-Complex molecules have more chem energy
-Complex molecules have more chem energy
11
New cards
heat
the transfer of this energy from one molecule/object to another
12
New cards
system
the matter under study
13
New cards
surroundings
anything outside of the system
14
New cards
open systems
Energy and matter can be transferred between the system and its surroundings
15
New cards
closed system
unable to exchange energy and matter with its surroundings
16
New cards
First Law of Thermodynamics
energy in the universe in constant; energy can’t be created or destroyed but it can be transferred and transformed
17
New cards
Second Law of Thermodynamics
when transferred or transformed usable energy decrease, because some is converted to thermal energy and released as heat, which is unable to do work; thus, the entropy increases
18
New cards
entropy
quantity that measures disorder or randomness; the more randomly arranged a collection of matter is, the greater its entropy
Heat energy has the most entropy because it is
random molecular motion
Heat energy has the most entropy because it is
random molecular motion
19
New cards
spontaneous process
a process that occurs without any outside input of energy or outside help
rarely happen and when they happen, takes years to happen though, which is why enzymes are preferred
rarely happen and when they happen, takes years to happen though, which is why enzymes are preferred
20
New cards
non-spontaneous process
a process that cannot occur without an input of energy or outside help
21
New cards
free energy
Portion of a system's energy that can perform work when temperature is uniform throughout the system. (G)
22
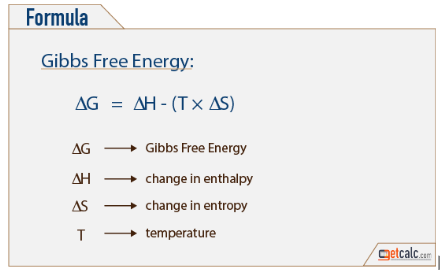
New cards
Know Gibbs Free energy formula and what each variable means
enthalpy: total energy in a biological system
23
New cards
What does it mean when the change in free energy is positive?
it means that there is an absorption of free energy
anabolic, endergonic
ex: photosynthesis (takes solar energy and invests into bonds of glucose molecule)
anabolic, endergonic
ex: photosynthesis (takes solar energy and invests into bonds of glucose molecule)
24
New cards
What does it mean when the change in free energy is negative?
it means that there is a release of free energy
catabolic, exergonic
ex: cellular respiration (breaks glucose bonds and energy stored becomes ATP)
catabolic, exergonic
ex: cellular respiration (breaks glucose bonds and energy stored becomes ATP)
25
New cards
Be able to relate the amount of free energy in a system to the stability and work capacity of the system
the more free energy in the bonds the less stable because it’s bigger, more organized, lower entropy
less free energy in bonds, more in external factors, more stable because smaller, less organized, higher entropy
less free energy in bonds, more in external factors, more stable because smaller, less organized, higher entropy
26
New cards
Endergonic reactions
intake of free energy, positive change in G
27
New cards
Exergonic reactions
release of free energy, negative change in G
28
New cards
What is the change in free energy when a cell has reached equilibrium?
no change in free energy
29
New cards
How do cells keep from reaching equilibrium?
cells are constantly moving and recycling things in and out of the cell; energy coupling
also na/k pumps
also na/k pumps
30
New cards
Chemical Work
The pushing of endergonic reactions that would not occur spontaneously, such as the synthesis of polymers from monomers
31
New cards
Transport Work
The pumping of substances across membrane against the direction of spontaneous movement
32
New cards
Mechanical Work
Beating of cilia, Contraction of muscle cells, and Movement of chromosomes during reproduction
33
New cards
energy coupling
The use of energy released by an exergonic process to drive an endergonic one; Mediated by ATP
34
New cards
structure of ATP
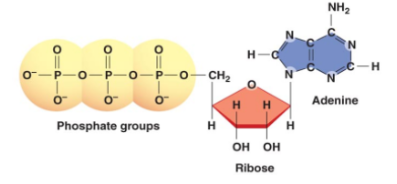
35
New cards
How much energy is released when ATP is hydrolyzed?
-7.3 kcal/mol (standard conditions)
-13 kcal/mol (cellular conditions)
-13 kcal/mol (cellular conditions)
36
New cards
Why is ATP such an unstable molecule?
The 3 phosphate groups all have an equal negative charge, and like charges repel. The phosphates want to break off so they are very unstable.
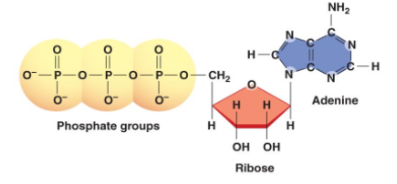
37
New cards
Explain how ATP drives cellular work through phosphorylation.
When ATP phosphorylates the phosphorylated intermediate (recipient of the terminal phosphate) the molecule becomes less stable with more free energy to do work
Transport work: The transfer of a phosphate to a transport protein changes its shape and.or its ability to bind with another molecule
Mechanical work involving motor proteins: ATP bonds to the motor protein, hydrolyzes, causes the motor protein to move, another ATP binds
Transport work: The transfer of a phosphate to a transport protein changes its shape and.or its ability to bind with another molecule
Mechanical work involving motor proteins: ATP bonds to the motor protein, hydrolyzes, causes the motor protein to move, another ATP binds
38
New cards
Describe the ATP cycle
Catabolic reactions fuel the phosphorylation of ADP to form ATP
energy comes from cellular respiration or photosynthesis
ATP is hydrolyzed and releases energy to do work in the cell
energy comes from cellular respiration or photosynthesis
ATP is hydrolyzed and releases energy to do work in the cell
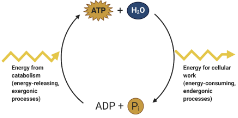
39
New cards
Why don’t most spontaneous processes happen spontaneously?
They take a very long time to actually happen, usually years
40
New cards
enzyme
A macromolecule that acts as a catalyst, a chemical agent that speeds up a reaction without being consumed
41
New cards
activation energy
the energy needed to reach the transition state, the point where the bonds can break
42
New cards
transition state
the state where the bonds of the reactants start to break
43
New cards
What do enzymes do to catalyze reactions?
lower their activation energy
44
New cards
substrate
The reactant(s) an enzyme acts on
45
New cards
Explain why there is such high specificity for an enzyme and its substrate.
Specificity of the enzyme / substrate interaction is determined by the shape of the active site.
Also R groups of amino acids on enzyme must be a complementary charge to the substrate
Also R groups of amino acids on enzyme must be a complementary charge to the substrate
46
New cards
active site
location on the enzyme that substrates bond to
47
New cards
induced fit
When a substrate binds to the active site, it “grabs” the substrate in a tight fit
48
New cards
catalytic cycle
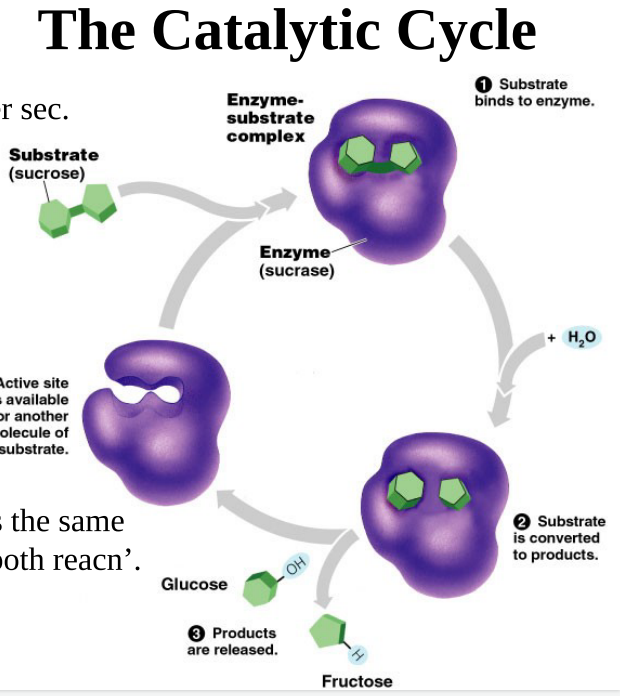
49
New cards
How do enzymes lower activation energy? (4 ways)
when an enzyme attached to substrate, the induced fit can stretch the substrate, which puts stress on the bonds and make them easier to break, thus lowering their activation energy
provide a template in which the substrate can come together, in a way that a reaction can occur between them
active site can't provide a microenvironment that is conducive to the reaction
-ex. active site can have acidic R-groups and some reactions do better in low pH environment
direct participation of the active site in the chemical reaction
-might involve brief covalent bonding between the substrate and side chains of the amino acids of the enzymes
provide a template in which the substrate can come together, in a way that a reaction can occur between them
active site can't provide a microenvironment that is conducive to the reaction
-ex. active site can have acidic R-groups and some reactions do better in low pH environment
direct participation of the active site in the chemical reaction
-might involve brief covalent bonding between the substrate and side chains of the amino acids of the enzymes
50
New cards
Explain how substrate concentration influences the rate of reaction
increase in substrate concentration, increase rate of reaction until enzymes run out to convert
decrease in substrate concentration, decrease rate, less substrate to convert
decrease in substrate concentration, decrease rate, less substrate to convert
51
New cards
Explain how temperature and pH (AND SALINITY) influence enzyme activity and reaction rates
-increase in temp: particles move around more and bump into one another more so reactions happen more frequently
too high, enzyme denatures
-decrease in temp: slows down because particles less active
-pH changes activeness and shape of the enzyme - optimal pH is best
too high, enzyme denatures
-decrease in temp: slows down because particles less active
-pH changes activeness and shape of the enzyme - optimal pH is best
52
New cards
cofactor
inorganic materials that help with enzymes catalyze reactions
-Zinc, Iron, copper
-Zinc, Iron, copper
53
New cards
Coenzyme
organic materials that help enzymes catalyze reactions; other proteins that aren't enzymes
54
New cards
competitive inhibitor
inhibitor that stops enzymatic activity by binding to the active site, blocking substrate
-cell can overcome competitive inhibition by adding more substrate
-cell can overcome competitive inhibition by adding more substrate
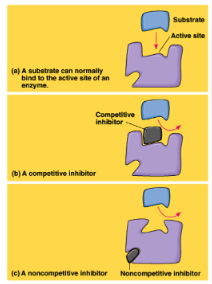
55
New cards
noncompetitive inhibitor
inhibitor that stops enzymatic activity by binding to a different site and changing the shape of the active site so the substrate can’t bind onto it
56
New cards
What is allosteric regulation?
-activator locks the enzyme in its active form when the -active sites are exposed so more substrate can be made into product
inhibitor locks the enzyme in an inactive form when the active site is not exposed
Changes by a conformational change of shape
inhibitor locks the enzyme in an inactive form when the active site is not exposed
Changes by a conformational change of shape
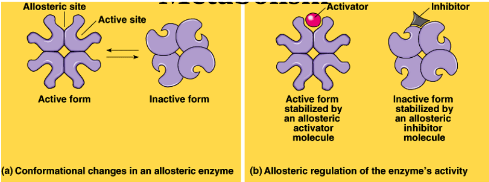
57
New cards
What is the structural level of most proteins that are regulated allosterically?
Quaternary since there are multiple units
58
New cards
Explain how ATP and ADP act as inhibitors and activators.
ATP is an inhibitor because it can’t allow the cell to do work immediately because it cannot be used as energy
ADP + P is an activator because it can be instantly used to do work (phosphorylation)
ADP + P is an activator because it can be instantly used to do work (phosphorylation)
59
New cards
cooperativity
Substrate molecules bind to one active site and stabilize the other active sites so they remain in the active form.
Substrates bound to one active site makes it easier for more substrates to bind to the other active site.
Substrates bound to one active site makes it easier for more substrates to bind to the other active site.
60
New cards
Explain how feedback inhibition can shut off a metabolic pathway
Enzymes can be turned off by feedback inhibition.
Metabolic pathways consist of a chain of reactions.
Enzyme 1 makes the first molecule in the chain.(intermediate A)
The final product (isoleucine)
Once enough product is made it diffuses back to the 1st enzyme.
Isoleucine binds to enzymes as a noncompetitive inhibitor because no more needs to be made.
Metabolic pathways consist of a chain of reactions.
Enzyme 1 makes the first molecule in the chain.(intermediate A)
The final product (isoleucine)
Once enough product is made it diffuses back to the 1st enzyme.
Isoleucine binds to enzymes as a noncompetitive inhibitor because no more needs to be made.
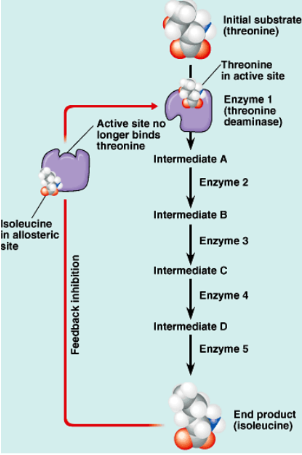
61
New cards
Enzyme structure/specificity essay
-EXPLAIN the enzymes active site and the substrate are similar in shape and charge
-the active site is the most important region
-substrate has to be complementary in shape and charge of the R-groups of amino acids in enzyme
-Enzymes are proteins, have levels of structure
-primary structure: dictates R-groups which determines charge, imwhat substrates its gonna react
-tertiary structure; determines shape of active site
-conformational change in shape
-EXPLAIN catalytic cycle: substrate binds to active site of enzyme; enzyme catalyzed reaction; does so by decreasing the activation energy needed to get to transition phase; products are released from the enzyme
enzyme active sites open for more substrates
-the active site is the most important region
-substrate has to be complementary in shape and charge of the R-groups of amino acids in enzyme
-Enzymes are proteins, have levels of structure
-primary structure: dictates R-groups which determines charge, imwhat substrates its gonna react
-tertiary structure; determines shape of active site
-conformational change in shape
-EXPLAIN catalytic cycle: substrate binds to active site of enzyme; enzyme catalyzed reaction; does so by decreasing the activation energy needed to get to transition phase; products are released from the enzyme
enzyme active sites open for more substrates
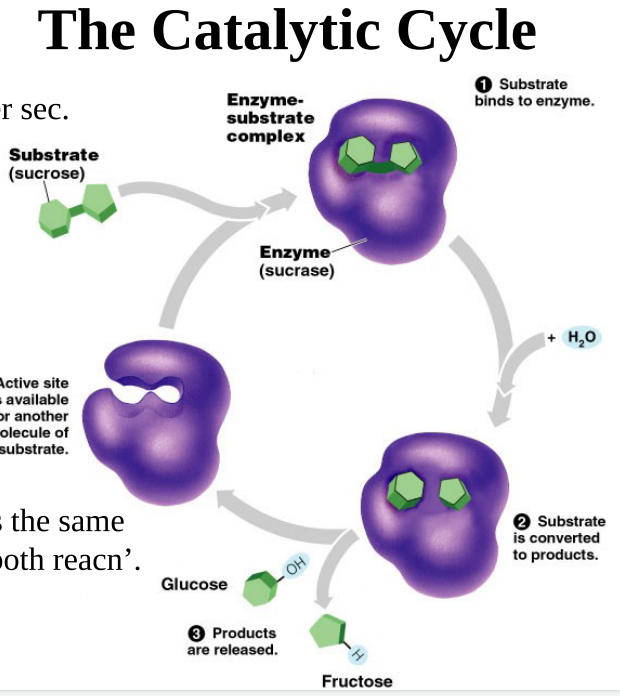
62
New cards
Explain the graph
-There is a certain amount of active sites for the increasing amount of substrate
-at first there's a lot of production because of the high enzyme to substrate ratio
-as substrate is added, there’s not enough enzymes to keep up with the inc in substrate so the rate slows
-Explain how would you increase the reaction rate: increase enzyme concentration to make more active sites available for substrate
-at first there's a lot of production because of the high enzyme to substrate ratio
-as substrate is added, there’s not enough enzymes to keep up with the inc in substrate so the rate slows
-Explain how would you increase the reaction rate: increase enzyme concentration to make more active sites available for substrate
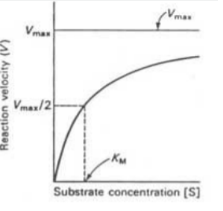
63
New cards
Explain the graph
-each enzyme has a certain optimal temperature
-as temp increases the particles move faster and bump into one another much more
-more reactions happen and as a result the rate of reaction increases exponentially
-the top of the curve is the max rate and the optimal temp
-as the temp goes over the optimal temp the protein starts to denature and can’t catalyze anymore
-optimal temp: the maximum point on the graph, the temp where the rate of reaction is highest
-as temp increases the particles move faster and bump into one another much more
-more reactions happen and as a result the rate of reaction increases exponentially
-the top of the curve is the max rate and the optimal temp
-as the temp goes over the optimal temp the protein starts to denature and can’t catalyze anymore
-optimal temp: the maximum point on the graph, the temp where the rate of reaction is highest
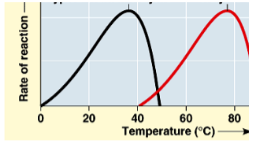
64
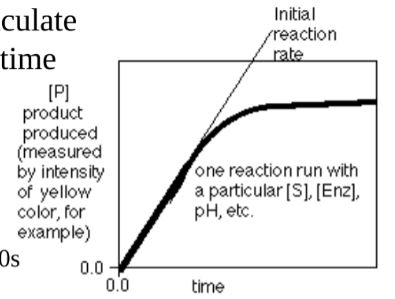
New cards
Explain the graph
-This graph shows the rate of product synthesis as a reaction progresses (with time). The first increase in rate shows the amount of product increasing and production occurs. The sudden plateau is when all the substrate is used and no more product can be produced.
-How would you calculate the rate for a given time interval: always y/x; product/time in this case
-How would you calculate the rate for a given time interval: always y/x; product/time in this case