set 4 - ph scale and buffer (unit 1)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
pH
a measure of h30- / h+
disasociation of water
rare - 2 out of 550 water molecule
h2o + h2o ---> h3o+ + OH-
ph of water
7
ph scale
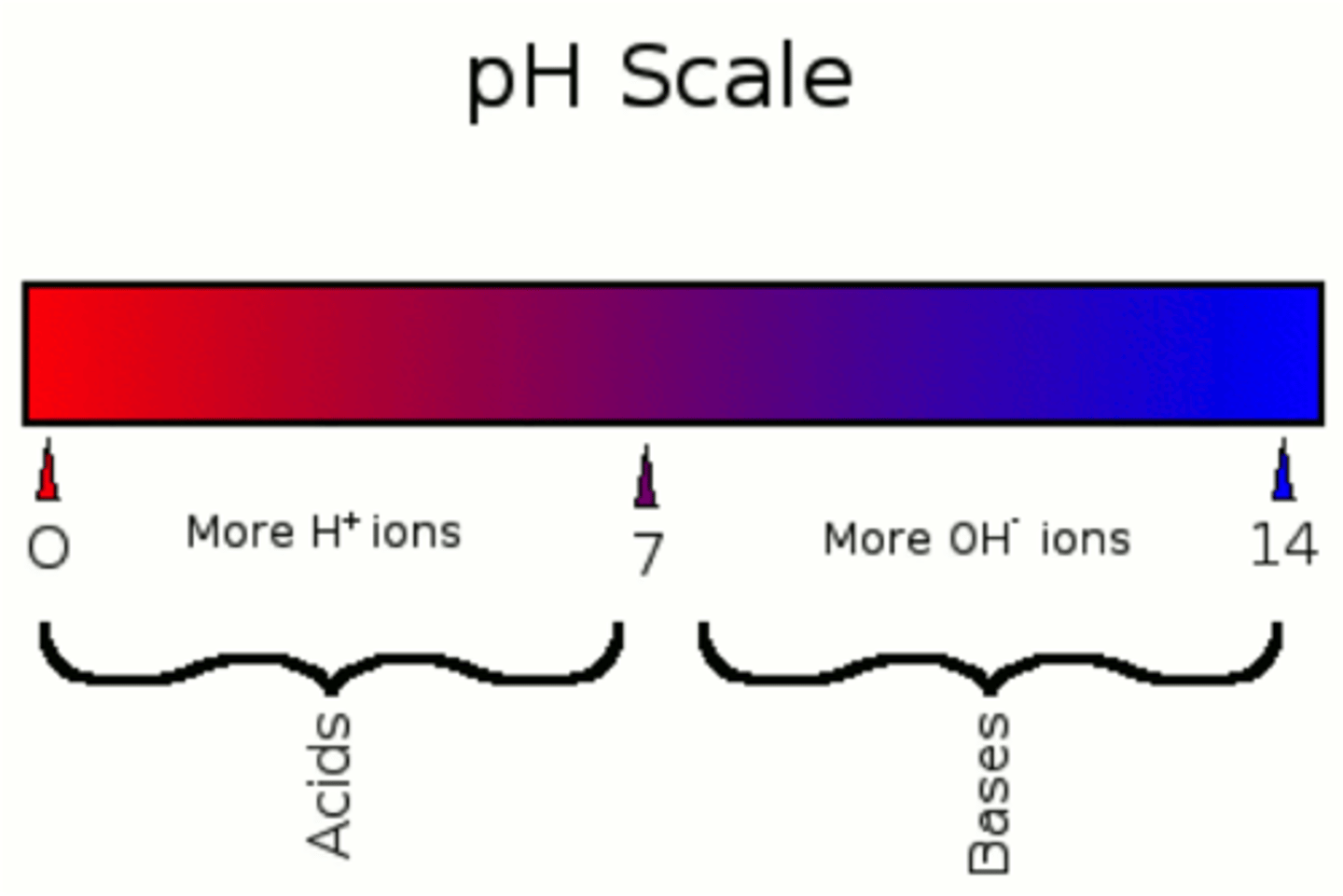
increasing acid means (in terms of ions)
more h3o + / h+ ions
decreasing acid means (in terms of ions)
less h3o + / h+ ions
acid
any substance that increase in hydronium
HCl + H2o --> H3O+ + cl-
base
any substance that decrease in hydronium
NH3 +H2o --> NH4 + OH-
ph of blood
7.2 = dead = acidosis
7.4 = perfect
7.8 = dead = alkalosis
effects of alkalosis
- overstimulation of nervous system
- muscle spasm
- contraction
effects of acidosis
- depression on nervous system
- fainintg
- weakness
- coma
ph can be described by
- respiratory problem
- not eating
- heavy exercise
- uncontrolled diabetes
- excess consumption of carbonic drinks
- malfunction of kidneys
- vomitting
- hyper ventilation
buffer
and how does it work
a substance that resists a change in Ph
by adding h + when needed
by accepting h+ when in aexcess
carbonate/ bicarbonate buffer
CO2 + H2O <--> H2CO3 <--> H+ + HCO3-
carbondioxide + water <--> carbonic acid <--> hydrogen ions + bicarbonation/ hydrogen carbonate
buffers in the human body
- kidneys and lungs are 2 organs that play an important role in Ph regulation
- protein act as a buffer and help to maintian the proper ph in blood and cells