MicroLab - Long quiz 1
1/161
Earn XP
Description and Tags
midterm topics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
162 Terms
biological safety levels
series of protections relegated to autoclave-related activities that take place in particular biological labs
individual safeguards designed to protect laboratory personnel and the surrounding environment and community.
they are important because they dictate the type of work practices that are allowed to take place in a lab setting
they heavily influence the overall design of the facility in question
centers for disease control and prevention (CDC)
sets BSL lab levels as a way of exhibiting specific controls for the containment of microbes and biological agents
the lab levels are determined by the following
risk related to containment
severity of infection
transmissibility
nature of work conducted
origin of the microbe
agent in question
route of exposure
biological safety level 1 (BSL-1)
lowest of the four
personnel work with low-risk microbes that pose little to no threat of infection in healthy adults
research taking place on benches without the use of special containment equipment
not required to be isolated from surrounding facilities
example:
non-pathogenic strains of E.coli
standard microbial practices of BSL-1
mechanical pipetting only (no mouth pipetting allowed)
safe sharps handling
avoidance of splashes or aerosols
daily decontamination of all work surfaces
hand washing
prohibition of food, drink, and smoking materials
personal protective equipment:
eye protection
gloves
lab coat/gown
biohazard sign
immediate decontamination after spills, infectious materials are also decontaminated prior to disposal (autoclave)
biological safety level 2 (BSL-2)
maintain the same standard microbial practices as BSL-1 labs
includes enhanced measures due to the potential risk of the aforementioned microbes
greater care to prevent injuries such as cuts and other breaches of the skin
practices required in a BSL-2 lab setting
appropriate personal protective equipment (PPE) must be worn
all procedures that can cause infection from aerosols or splashes are performed within a biological safety cabinet (BSC)
an autoclave or an alternate method of decontamination is available
the laboratory has a self-closing, lockable doors
a sink and eyewash station should be available
biohazard warning signs
access to the lab is more restrictive than BSL-1 lab
biological safety level 3 (BSL-3)
includes work on microbes that are either indigenous or exotic, can cause serious or potentially lethal disease through inhalation
work is often strictly controlled and registered with the appropriate government agencies
laboratory personnel are also under medical surveillance and could receive immunization for microbes they work with
examples:
yellow fever
west nile virus
bacteria that causes mycobacterium tuberculosis
bacteria:
yersinia pestis
brucella abortus
chlamydia psittaci
pseudomonas mallei
viruses:
west nile fever
herpes B
hepatitis A
common requirements in a BSL-3 laboratory
standard personal protective equipment must be worn, respirators might be required
solid-front wraparound gowns, scrub suits, or coveralls are often required
all work with microbes must be performed within an appropriate BSC
access hand-free sink and eyewash stations near the exit
sustained directional airflow to draw air into the laboratory from clean areas towards potentially contaminated areas
a self-closing set of locking doors with access away from general building corridors
access to BSL-3 labs must be restricted and controlled at all times
biological safety level 4 (BSL-4)
rare
highest level of biological safety
consist of work with highly dangerous and exotic microbes
infections caused by these types of microbes are frequently fatal, and come without treatment or vaccines
laboratory is extremely isolated - located in a separate building or an isolated and restricted zone of the building
examples:
ebola
marburg viruses
BSL-4 laboratories have the following containment requirements
personnel are required to change clothing before entering, shower upon exit
decontamination of all materials before exiting
personnel must wear appropriate personal protective equipment from prior BSL levels, as well as a full body, air-supplied, positive pressure suit
a class-III biological safety cabinet
isolation of bacteria
A primary method used to separate different groups of microorganisms.
A technique that distinguishes groups of bacteria based on their growth patterns.
Bacteria grow differently on various nutrient media depending on temperature, pH, and oxygen availability.
Isolation of bacteria is essential for their identification and classification.
The isolation process involves specimen collection, preservation, culturing, and microscopic examination.
Specimens can come from clinical, environmental, or food samples.
Specimens must be preserved under sterile conditions and transported quickly to maintain bacterial viability.
Both culture and non-culture methods are used for bacterial isolation.
In culture methods, bacterial growth is indicated by turbidity or colony formation in liquid or solid media.
Non-culture methods like PCR and LCR are used to detect and identify bacteria.
Microscopic examination after culturing and staining helps identify bacteria by color, shape, size, and other features.
Bacterial isolation techniques help define and differentiate various bacteria
methods of isolation of bacteria
pouring method
spreading method
streaking method
serial dilution method
pouring method
simplest method
a bacteria suspension laden with a huge bacterial population is generally taken
procedure for pouring method
Take 1 mL of the bacterial sample and place it into a sterile Petri dish.
Bacterial growth requires a nutrient source such as carbon or nitrogen.
Prepare and pour nutrient agar medium into Petri plates containing the bacterial sample.
Rotate the plates clockwise and counterclockwise to evenly distribute the sample and medium.
Allow the culture plates to solidify before incubation.
Incubate the plates at 35–37°C for up to 48 hours for optimal bacterial growth.
After incubation, visible bacterial colonies will appear.
spreading method
a very simple method to perform bacterial isolation
procedure for spreading method
The nutrient medium is poured into sterile Petri plates before adding the bacterial sample.
Allow the nutrient medium in the Petri plates to solidify.
After solidification, add 1 mL of bacterial suspension onto the surface of the medium.
Use a T- or L-shaped spreader to evenly distribute the bacterial suspension.
Incubate the culture plates at 35–37°C for 24–48 hours.
After incubation, several bacterial colonies will appear.
The spread plate method is not commonly used for isolating pure cultures.
streaking method
a very popular and most widely used method for the isolation of pure culture
limited population of bacteria in the streaking method, pure culture isolation is quite easier than the pour plate and spread plate method
we can culture, isolate, and study the individual colony of bacteria
procedure for streaking method
Pour freshly prepared nutrient agar into sterile Petri plates and allow it to solidify.
Sterilize the inoculating loop by heating it over a flame until red hot.
Using the sterilized loop, collect the inoculum and streak it over the solidified nutrient agar while keeping the plate near the flame to prevent contamination.
Incubate the streaked culture plates at 35–37°C for 24–48 hours.
serial dilution method
known for the isolation and culturing of bacteria
procedure for the serial dilution method
Prepare serial dilutions of the bacterial suspension in successive test tubes.
Transfer 1 mL of the sample sequentially to each test tube in the dilution series (10⁻¹, 10⁻², 10⁻³, etc.).
Inoculate the diluted samples using one of the three methods: pour plate, streak plate, or spread plate.
Serial dilution makes it easier to isolate bacteria from a smaller bacterial population.
More concentrated samples (10⁻¹) produce more colonies, while more diluted samples (10⁻⁴) produce fewer colonies.
Less diluted samples have a higher bacterial concentration, whereas more diluted samples have more water than bacteria.
Samples with fewer bacteria yield fewer colonies, and chosen colonies are stained and examined under a microscope for identification.
microscope
most important tool in microbiology research and studies
bright field microscopy
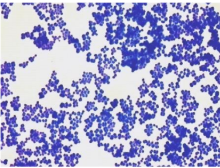
dark field microscopy
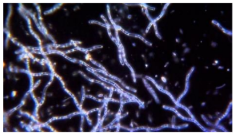
phase contrast microscopy

fluorescence microscopy
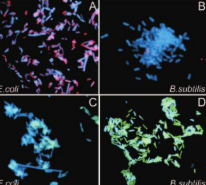
electron microscopy

parts of a microscope
arm
base
ocular / eyepiece
body tube
revolving nosepiece
objective lenses
stage
stage clips
diaphragm
light source / illuminator
coarse adjustment knob
fine adjustment knob
arm
supports the body tube and connects it to the base
base
serves as the microscope’s foundation and provides stability
ocular / eyepiece
the lens looked through to see the specimen
body tube
holds and aligns the eyepiece to the objective lenses
revolving nosepiece
holds the objective lenses and allows you to switch between them
objective lenses
provide different levels of magnification for viewing the specimen
stage
the platform where the slide is placed
stage clips
holds the slides in place
diaphragm
controls the amount of light that reaches the specimen
light source / illuminator
provides light to make the specimen visible
coarse adjustment knob
moves the stage up and down for focusing
fine adjustment knob
adjusts the focus precisely for a clear image
scanner objective lens
red
4x magnification
low power objective (LPO)
yellow
10x magnification
high power objective (HPO)
blue
40x magnification
oil immersion objective (OIO)
white
100x
uses oil:
cedar oil
mineral oil
has the same refractive index as the mirror
colony form
overall shape of the colony
circular, irregular, filamentous
colony edge/margin
the structure of the colony on its edges that is exposed to air
entire, undulate, filiform, lobate, curled, scalloped, and serrated/erose
one type of colony that has a distinct filiform edge is Bacillus anthracis
colony elevation
defined as the shape displayed by a colony when overserved from the side
flat, raised, convex, pulvinate, umbonate, crateriform
colony size
important in fungal and bacterial colony morphology
described in millimeters
punctiform, small, medium, large
colony chromogenesis / color / pigmentation
a colony can display a singular color but have different tones and hues from the middle to the outer part
the color seen on an agar plate may not be the actual color of the bacteria but the pigment that is produced due to the immersion of the bacteria in the media
it is crucial to note the opacity of an observed colony
transparent, translucent, opaque, or iridescent
colony surface and consistency
texture and consistency of the bacteria
surfaces are described as shiny, dull, smooth, rough, veined, glistening, wrinkled, etc.
consistency or texture us often described after touching or scraping on the colony
dry, brittle, mucoid, butyrous, viscid
hemolysis
on blood agar, the pattern of red blood cell lysis around the colony
can be complete = beta-hemolysis
can be partial = alpha-hemolysis
growth media / media
substances where microbes are grown
provides the nutrients necessary to sustain the metabolic activities and reproduction of the microbes
can be liquid or solid
broth
liquid media
used to determine growth patterns in a liquid medium
the method of choice for growing large quantities of bacteria
agar
solid growth media
a mixture of polysaccharides derived from red algae
solidification agent
it id not broken down by bacteria
contains no nutrients that can be used by bacteria
melts at high temperatures
agar plates, agar slants, agar deeps
stocks
because of the relatively small tube opening and the surface area for growth, agar slants are commonly used and store in bacteria for intermediate period of time
pure culture
a culture that contains a single microbial species
when left unintended, the culture becomes contaminated
aseptic technique
a collection of procedures and techniques designed to prevent the introduction of unwanted organisms into a pure culture or into the lab environment
meaning of aseptic
without contamination
sterilization
complete removal of all vegetative cells, endospores, and viruses
all media which the cells are grown in are sterilized by an autoclave
autoclave
uses moist heat/steam under pressure to destroy all life forms
most vegetative cells can be killed at temperatures between 60 to 80 C while bacterial spores require temperatures above boiling
needs at least 20 minutes to kill all spores as well as vegetative cells
disinfection
the killing or growth inhibition of vegetative microbes
chemical disinfectants (chlorine, bleach, etc.) are used to clean non-living surfaces
antiseptics
antimicrobial chemicals safe for use on living skin or tissues
example: hydrogen peroxide and isopropyl alcohol
solid media
for isolation of bacteria as a pure culture on a solid medium
Robert Koch
agar is used for hardening the media at 1.5-2.0% concentration
allows the growth of bacteria as colonies by streaking on the medium
solidified at 37 degrees Celsius
growth of bacteria on solid mediums appear smooth, rough, mucoid, round, irregular, filamentous, punctiform
examples:
nutrient agar
MacConkey agar
blood agar
chocolate agar
semi-solid media
shows the motility of the bacteria and the cultivation of microaerophilic bacteria
has an agar concentration of 0.5% or less
has a jelly consistency
the bacterial growth in semi-solid medias appear as a thick line in the medium
examples:
Stuart’s and amies media
hugh and leifson’s oxidation fermentation medium
mannitol motility media
liquid media
shows the growth of a large number of bacteria
broth that allows bacteria to grow uniformly with turbidity
growth occurs at 37 degrees Celsius in an incubator for 24 hours
for fermentation studies
bacterial growth in liquid medias — turbidity is seen at the end of the broth
examples:
nutrient broth
tryptic soy broth
MR-VP broth
phenol red carbohydrate broth
basal media
enhances thee growth of many microorganisms
routinely used medium in the lab, having carbon and nitrogen
allows the growth of non-fastidious bacteria without any enrichment source
Staphylococcus and Enterobacteriaceae grow in this media
Nutrient agar and peptone water
enriched media
requires the addition of other substances life blood, egg, or serum
allows the growth of devised microorganisms but inhibits other and fastidious microbes grow as they require nutrients like vitamins and growth-promoting substances
blood agar, chocolate agar, LSS, monsor’s taurocholate, lowenstein jensen media
selective media
shows the growth of selective microbes or desired microorganisms
inhibits the growth of unwanted microbes
inhibition occurs by adding bile salts, antibiotics, dyes, and pH adjustments
enrichment media
a liquid media that enhances the growth of desired bacteria even at a low density while inhibiting unwanted bacteria
isolation of soil or fecal microorganisms
F-broth (salmonella typhi from fecal samples)
indicator or differential media
contains indicators that show visible changes to differentiate bacteria based on biochemical reactions
examples:
mannitol salt agar = yellow colonies —> mannitol fermenters
blood agar = distinguishes hemolytic vs. non-hemolytic bacteria
macConkey agar = pink colonies —> lactose fermenters (pale = non-lactose)
transport media
maintains the viability of microorganisms during transport without allowing their multiplication
examples:
stuart’s trasnport medium - lacks nutrients to prevent overgrowth
cary-blair medium - for fecal samples (cholera)
pike’s medium - for streptococci from throat samples
storage media
used to maintain and preserve bacterial cultures for long periods
examples
cooked meat broth
nutrient agar egg saline
aerobic media
supports the growth of microorganisms that require oxygen
anaerobic media
supports the growth of anaerobic bacteria by maintaining low oxygen levels
assay media
used to test potency of vitamins, amino acids, or antibiotics
minimal media
a defined medium with minimal nutrients required for growth of wild-type microorganisms
used to differentiate wild-type from mutant cells and selects recombinants
fermentation media
provides nutrients for microbial growth and production of fermentation productions
components:
major: carbon and nitrogen
minor: minerals, vitamins, buffers, anti-foaming agents, etc.
resuscitation culture media
specialized medium for reviving stressed or injured bacteria that have lost the ability to grow under normal conditions
preparation of a bacterial smear
Label the slide – Use a grease pencil to mark one end of the slide with the bacterial culture name.
Prepare the sample – Place a drop of normal saline on a clean slide and add a small amount of bacteria (from solid or broth culture).
Spread the smear – Evenly spread the bacteria to form a thin layer, leaving space on all sides for viewing.
Air dry – Allow the smear to dry at room temperature (25–28°C) in a dust-free area.
Heat-fix the smear – Pass the slide (smear side up) through a flame three times or use 70% alcohol for 2 minutes to fix the bacteria.
Avoid overheating – Overheating may burn or distort cells, leading to poor staining results.
Stain the smear – Apply an appropriate stain on the fixed smear using a staining rack, depending on the staining technique used
Gram staining
Hans Christian Gram in 1884
differentiate bacteria into gram positive and gram negative groups based on their cell wall structures
some bacteria retain the primary stain (usually crystal violet) due to their thick peptidoglycan layer
other bacteria lose the primary stain during decolorization and show the counterstain (safranin = red)
gram staining procedure
Crystal violet – Primary stain; colors all cells purple.
Iodine – Acts as a mordant to fix the dye.
Alcohol (ethanol) – Decolorizer; removes stain only from Gram-negative cells.
Safranin – Counterstain; colors Gram-negative cells pink.
results from gram staining
Gram-positive bacteria
Thick peptidoglycan layer, no outer membrane.
Retain crystal violet and appear purple.
Gram-negative bacteria
Thin peptidoglycan layer, has an outer membrane that dissolves in alcohol.
Lose crystal violet, take up safranin, and appear pink/red.
acid-fast staining (ziehl-neelsen method)
used to identify acid-fast bacteria that cannot be stained by the gram stain due to their waxy cell wall
Mycolic acid makes the bacterial cell wall waxy and impermeable to most stains.
Heat softens the wax, allowing the primary dye (carbol fuchsin) to enter the cells.
Acid-alcohol is used as a decolorizer:
Acid-fast cells resist decolorization and retain the red dye.
Non–acid-fast cells lose the dye and are later stained blue with methylene blue
acid-fast staining procedure
Primary stain: Carbol fuchsin (red)
Heat: Helps the dye penetrate the waxy cell wall
Decolorizer: Acid–alcohol
Counterstain: Methylene blue
results from acid-fast staining
Acid-fast bacteria (AFB): Bright pink/red (retain carbol fuchsin)
Non–acid-fast bacteria: Blue (take up methylene blue)
endospore stain
endospores = dormant, non-reproductive structures formed by gram-positive bacteria
allows the bacteria to withstand harsh environmental conditions
makes endospores visible under a bright background since they resist normal stains
endosporulation
DNA replication occurs in the bacterial cell.
Layers of peptidoglycan and protein form around the DNA.
The endospore matures and is released from the cell.
It can remain dormant for years until conditions become favorable again.
When conditions improve, it germinates into a vegetative (active) cell.
endospore staining procedure
Primary stain: Malachite green (water-soluble; penetrates spores with heat).
Heat: Acts as a mordant to drive the dye into the spore coat.
Decolorizer: Water removes the dye from vegetative cells.
Counterstain: Safranin stains the vegetative cells pink/red
result for endospore staining
Endospores: Green
Vegetative cells: Pink/red
how to solve vsp/cmu

what if the given plated medium is only 0.1 ml?
multiply by 10
multiply to the reciprocal of the test tube it came from
what if the given plated medium is only 0.2 ml?
multiply by 5
multiply to the reciprocal of the test tube it came from
culture
increase in the population of bacterial cells
binary fission
the prevailing means of bacterial reproduction
division exactly in half
most common means of bacterial reproduction
forming two equal size progeny
genetically identical offspring
cells divide in a geometric progression doubling cell number
doubling time is the unit of measurement of microbial growth
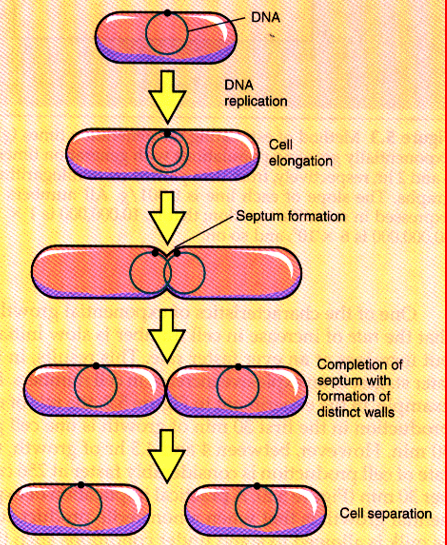
generation time
the time it takes cells to divide and double in population
generation time of Escherichia coli
15-20 minutes
generation time of Staphylococcus aureus
20-30 minutes
generation time of Salmonella enteritidis
20-30 minutes
generation time of Mycobacterium tuberculosis
15-20 hours
generation time of Lactobacillus acidophilus
66-67 minutes