Dalton's Law of Definite proportions
1/12
Earn XP
Description and Tags
dalton's atomic model contained the idea that elements (kind of atoms) combine in simple whole number ratios to form compounds. The law of constant composition says that a compound is defined by a particular elemental atomic ratio which does not change. these atomic ratios can be studied by measuring the mass rations in chemical reacitons.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
in compounds, elements combine in
whole number ratios
ratio does not change
ratio partially defines a compound
ionic formula tells
ratio of elements
molecular formula tells
ratio of elements
composition of molecule
law of definite proportions
an empirical law that states elements will combine in specific, observable, mass ratios to form compounds
formula of determining a percentage of an element in a compound
(Melement/Mcompound) x 100
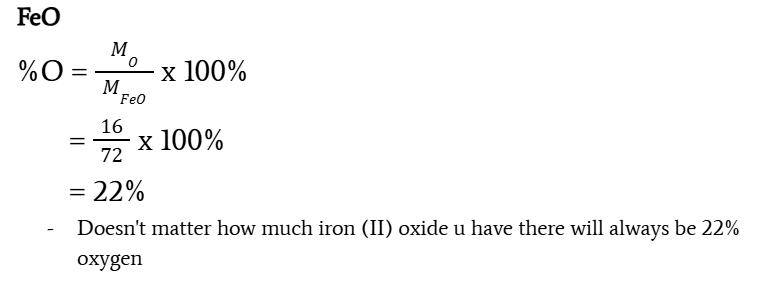
determine the percentage (by mass) of oxyegn in each of the oxides: FeO and Fe2O3
Iron (II) oxide: 22%
Iron (III) oxide: 30%

formula of determining percentage of element produced based on mass
Mcompound/Melement = x/mass
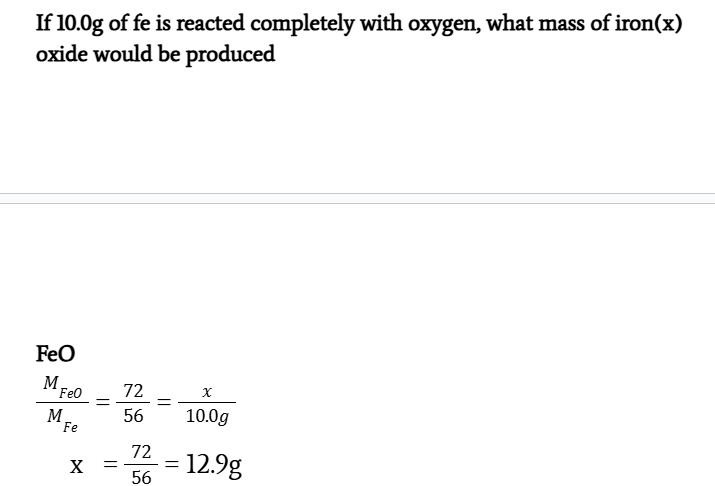
If 10.0g of Fe is reacted completely with oxygen, what mass of iron(x) oxide would be produced
MFeO/MFe = 72/56 = x/10.0
x = 72/56 × 10.0 g
x = 12.9 g
Fe2O3 = 14.3 g

percentage composition of a compound
what percentage of each element in a compound, calculate all then make sure they add up to 100%
Find the percentage composition of ascorbic acid (C6H8O6)
C6H8O6
Molecular mass:
MC6H8Og = 6(12.011amu) + 8(1.00794amu) + 6(15.9994amu)
= 176.12592amu
%C = 6MCMC6H8O6 x 100%
= 6(12.011amu)176.12592amu x 100%
= 40.9%
%H = 8MHMC6H8O6 x 100%
= 8(1.00794amu)176.12592amu x 100%
= 4.57%
%O = 6MOMC6H8O6 x 100%
= 6(15.9994amu)176.12592amu x 100%
= 54.5%
Therefore ascorbic acid is 40.9% carbon, 4.57% hydrogen and 54.5% oxygen by mass.

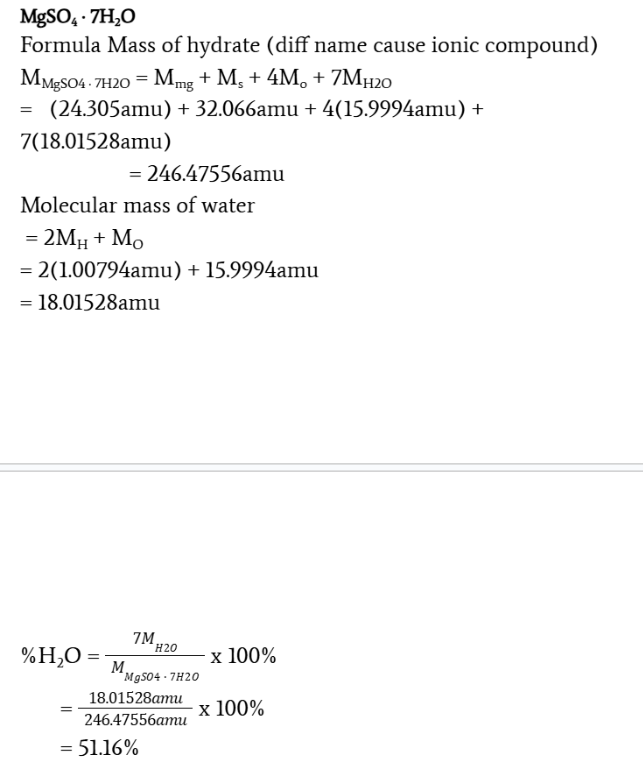
Determine the percentage composition of water in magnesium sulfate heptahydrate
MgSO4 · 7H2O
MMgSO4 · 7H2O = Mmg + Ms + 4Mo + 7MH2O
= (24.305amu) + 32.066amu + 4(15.9994amu) + 7(18.01528amu) = 246.47556amu
Molecular mass of water
= 2MH + MO = 2(1.00794amu) + 15.9994amu = 18.01528amu
% H2O = 7MH2O/MMgSO4 · 7H2O x 100% = 18.01528amu246.47556amu x 100% = 51.16%
molecular mass
referring to mass of an entire molecule, ex. MH2O
formula mass
referring to mass of an ionic compound, ex. MMgSO4