Covalent Bonding and Molecular Shape
1/43
Earn XP
Description and Tags
Functional Groups, Bonds,
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
formula for calculating formal charge
group # - dots - sticks
non-polar covalent bond
bond between two non-metals where electrons are shared equally
polar covalent bonds
bonds between two non-metals where electrons are shared inequally
what is the importance of functional groups?
allow for organization of organic molecules, sites of characteristic chemical reactions, and a basis for naming organic compounds
alkane
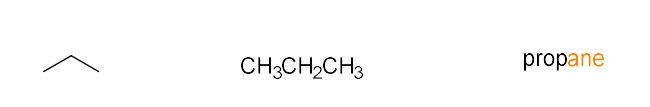
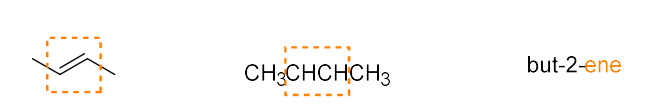
alkene
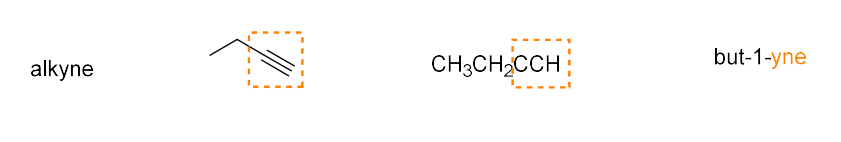
alkyne
aromatic (arene)
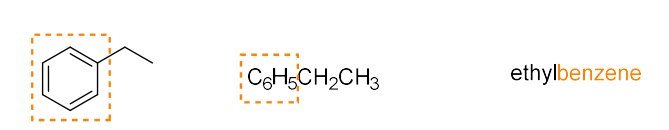
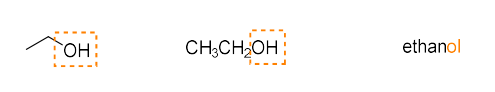
alcohol
thiol
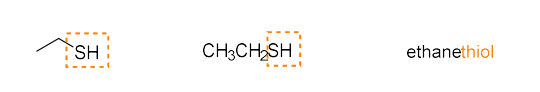
amine
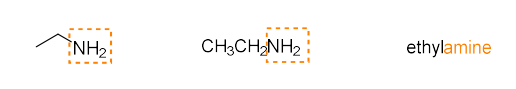
halide
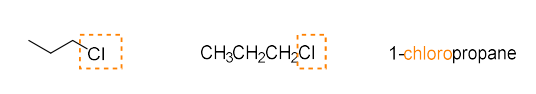
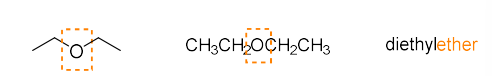
ether
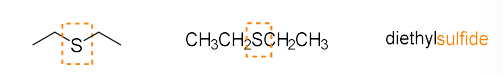
sulfide
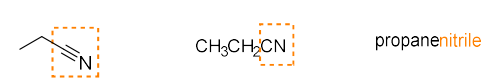
nitrile
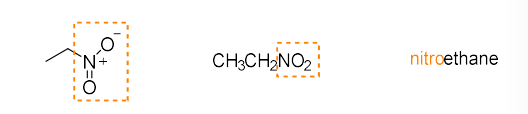
nitro
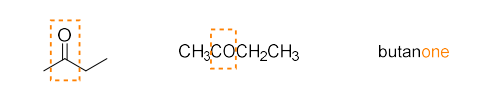
ketone
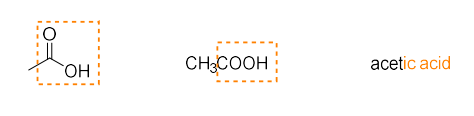
carboxylic acid
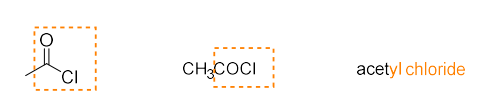
acid chloride
aldehyde

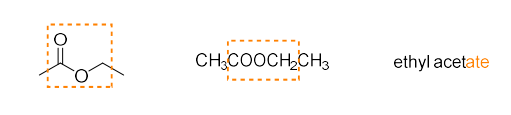
ester
amide
Explain primary, secondary, tertiary, and quaternary carbons
Primary carbons are bonded to one carbon, secondary two, etc…
Explain primary, secondary, and tertiary alcohols
Primary Alcohols: One carbon is bonded to another carbon
Secondary Alcohol: One carbon is bonded to two other carbons
Tertiary Alcohols: One alcohol is bonded to three other carbons
Explain primary, secondary, and tertiary amines
Primary Amines: Nitrogen bonded to one carbon
Secondary Amines: Nitrogen is bonded to two carbons
Tertiary Amines: Nitrogen is bonded to three carbons
What geomtry can carbon take on?
Linear (180/sp), Trigonal Planar (120, sp2), and tetrahedral (109.5, sp3)