chem1013: exam 2 (end of chapter 3, chapter 4, chapter 5, chapter 6)
1/172
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
173 Terms
What is mass percent composition?
each element’s percentage of total compound’s mass
We find the empirical and molecular formulas of a compound if what two things are known?
mass percent composition and molar mass
What is the formula of mass percent of an element?
[(mass of the element in 1 mol of compound) / (total mass of one mole of the compound)] x100
Calculate the mass percent composition of Cl in CCl2F2.
To calculate the mass percent composition of Cl in CCl2F2, divide the mass of Cl in one mole of CCl2F2 by the total mass of one mole of CCl2F2, then multiply by 100.
2(molar mass Cl)
_______________ X 100
molar mass CCl2F2
=[2(35.453 g/mol)] / [12.011 + 2(35.453) + 2(18.998)] X 100
= [70.906] / [120.911] X 100 = 58.7%
How can we use mass percent as a conversion factor?
since mass percent = per hundred, there are (mass percent in grams) per 100 grams of compound
ie: Cl is 58.7% mass of CCl2F2 → 58.7 g Cl : 100g CCl2F2
This allows us to convert between grams of an element and grams of the compound.
What do conversion factors tell us of an element in a compound?
ie: 1 mol CCl2F2 has 2 mol Cl atoms, thus, 1 mol CCl2F2 : 2 mol Cl
This means that for every mole of the compound, there are a specified number of moles of the element, allowing for stoichiometric calculations.
If you want to use the chemical formula as a conversion factor, what must you do first?
convert from grams to moles; the chemical formula indicates mol→ mol ratios, not mass;
therefore, you need to know the molar mass of the compound to make the conversion.
If we do not know the chemical formula of a compound, but know the percent mass of each element in the compound from experimental data, what can we determine?
empirical formula
What are the steps to calculate the empirical formula?
Convert the percent composition of each element to grams.
Convert grams to moles for each element.
Divide the moles of each element by the smallest number of moles to find the simplest ratio.
If necessary, multiply the ratio by a whole number to get integer subscripts.
What are the steps to calculate the molecular formula?
Determine the empirical formula.
Calculate the molar mass of the empirical formula.
Divide the molar mass of the compound by the molar mass of the empirical formula.
Multiply the subscripts in the empirical formula by this ratio to find the molecular formula.
molar mass= molar mass of empirical formula x n
find n
multiply subscripts in empirical formula by n
What is combustion analysis and it is used to find what kind of empirical formulas?
the unknown compound undergoes combustion in the presence of pure oxygen to produce carbon dioxide and water, allowing for the determination of the empirical formula by measuring the amounts of these products.
What are organic compounds?
composed of carbon, hydrogen, and a few other elements such as nitrogen, oxygen, and sulfur
What are hydrocarbons?
compounds composed of carbon and hydrogen only
What are alkanes?
single bonds
What are alkenes?
double bonds
What are alkynes?
triple bonds
All other organic compounds can be thought of as hydrocarbons with 1 or more ______— characteristic atoms or groups of atoms
functional groups
What is the prefix 1 for hydrocarbons?
meth-
What is the prefix 2 for hydrocarbons?
eth-
What is the prefix 3 for hydrocarbons?
prop-
What is the prefix 4 for hydrocarbons?
but-
What is the prefix 5 for hydrocarbons?
pent-
What is the prefix 6 for hydrocarbons?
hex-
What is the prefix 7 for hydrocarbons?
hept-
What is the prefix 8 for hydrocarbons?
oct-
What is the prefix 9 for hydrocarbons?
non-
What is the prefix 10 for hydrocarbons?
dec-
What is the affix for single bonds of hydrocarbons?
-ane
What is the affix for double bonds of hydrocarbons?
-ene
What is the affix for triple bonds of hydrocarbons?
-yne
How do you name simple, straight-chain hydrocarbons?
identifying the number of carbon atoms and adding the appropriate suffix (-ane, -ene, or -yne) based on the bonding type.
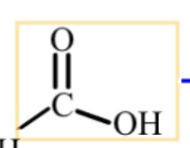
carboxylic acid

amine
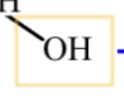
alcohol
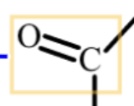
ketone
What is a chemical reaction?
1+ substances are converted to 1+ different ones, represented by a chemical equation
What must a chemical equation be, according to the law of conservation of mass?
balanced, with equal numbers of each type of atom on both sides
How are states specified using abbreviations in parentheses?
States are specified as (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous.
What can you change and what can you not change when balancing equations?
You can change the coefficients in front of compounds, but you cannot change the subscripts within a chemical formula.
What are the steps to balance chemical equations?
write a skeletal equation (unbalanced)
count # atoms on each side of the arrow
use whole number coefficients to balance
*start with the most complex compound and leave the pure for last
check for lowest whole number coefficients
What is reaction stoichiometry?
numerical relationships between reactants and products in a balanced chemical equation
What does reaction stoichiometry help us predict?
The amount of product formed from a given amount of reactant, or how much of 1 reactant is required to react with a given amount of another, in a chemical reaction.
What do coefficients specify?
relative amounts in moles of each substance
What is theoretical yield?
amount of product that can be made based on the limiting reagent
What is limiting reagent?
reactant that is completely consumed; limits product amount
What is excess reagent?
reactant still left over after reaction is complete
What is actual yield?
amount of product actually produced
What is the percent yield formula?
Percent yield = (actual yield / theoretical yield) x 100%
What are the steps to find theoretical yield, limiting reactant, excess reactant(s), and percent yield?
convert reactants to grams of the product asked in the equation
theoretical yield= grams of product formed based on limiting reactant
Limiting reactant= least amount of grams of product
Excess reactant= the reactant that remains after the reaction in complete
Find the percent yield by:
dividing actual yield (usually given in problem) by theoretical yield calculated
multiply by 100%
In this experiment, 28.6kg of C reacted with 88.2kg of TiO2, and 42.8kg of Ti was produced. What is the theoretical yield (in kg) of Ti, and what is the percent yield for this reaction? Also, identify the limiting and excess reagents.
To determine the theoretical yield of titanium (Ti), convert the masses of carbon (C) and titanium dioxide (TiO2) to Ti using stoichiometry based on the balanced reaction equation. The limiting reactant will be the one that produces the least amount of Ti, while the excess reagent is the one that remains after the reaction is complete. Percent yield is calculated by dividing the actual yield (42.8 kg) by the theoretical yield and multiplying by 100%.
What is a solution?
homogeneous, uniform mixture of 2 substances
What is a solvent?
majority component (often water)
What is a solute?
minority component
What is an aqueous solution?
solvent is water
What is a dilute solution?
small amount of solute relative to the solvent
What is a concentrated solution?
large amount solute relative to solvent
What can solution concentration be expressed as?
Molarity (M)
What is the formula for molarity (M)?
M = moles of solute / liters of solution
What is the molarity of a solution with 25.5 g KBr dissolved in enough water to make 1.75 L of solution?
To find the molarity, first calculate the moles of KBr by using its molar mass (119.00 g/mol). Molarity (M) is then calculated as moles of solute divided by the volume of solution in liters. Therefore, M = (25.5 g / 119.00 g/mol) / 1.75 L = 0.123 M.
What volume (in mL) of a 0.155 M KCl contains 2.55 g KCl?
To determine the volume in milliliters, first calculate the moles of KCl using its molar mass (74.55 g/mol). Then, apply the molarity formula (M = moles of solute / liters of solution) to find the volume in liters, and convert to milliliters. Thus, volume = (2.55 g / 74.55 g/mol) / 0.155 M = 220 mL.
What are stock solutions?
concentrated forms of solutions
How do we use stock solutions for our experiments?
we add solvent (usually water) to increase the volume and decrease the concentration (amount of solute stays the same); simple, we are diluting the stock solution to the desired concentration for use in experiments.
What equation do we use for dilutions, and what are the rules for this formula?
M1V1 = M2V2
M1 = initial concentration (stock)
V1 = initital volume
M2 = final concentration
V2 = final volume
volume units must match
concentration units must match
A lab procedure calls for 3.00 L of a 0.5 M CaCl2 solution. How should we prep this solution from a 10.0 M stock solution?
To prepare 3.00 L of a 0.5 M CaCl2 solution from a 10.0 M stock solution, use the dilution formula M1V1 = M2V2. Calculate V1 as follows: V1 = (M2V2) / M1. = (0.5 M)(3.00 L) / 10.0 M = 0.150 L (150 mL) of stock solution.
What is the concentration of a solution prepared by diluting 56.6 mL of a 2.25 M stock solution with enough water to make 1.05 L of solution?
To find the concentration, use the dilution formula M1V1 = M2V2. Here, M1 = 2.25 M, V1 = 56.6 mL (0.0566 L), and V2 = 1.05 L. Solving for M2, we find M2 = (M1V1)/V2 = (2.25 M)(0.0566 L)/(1.05 L) = 0.121 M.
What can we calculate with the volume and concentration of a reactant or product?
its number of moles— using the formula: moles = concentration × volume.
* to convert between species, we use molar ratios from the equation
When ionic solutes form a solution with water, what do ions undergo?
hydration (water molecules surround each ion)
What is dissociation?
solute ions break their attraction to each other and are attracted to the water molecules and disperse into the water (solvent)
ie: NaCl (s) → Na+ (aq) + Cl- (aq)
What happens to molecular solutes when they form solutions with solvents?
molecular solutes can also form solutions with solvents, but molecules only separate from each other (no dissociation, no bonds breaking)
ie: CH3OH (l) → CH3OH (aq)
What are electrolytes?
Substances that dissociate into ions in solution, conducting electricity.
What are strong electrolytes?
completely dissociate into ions when dissolved in water
mostly ionic compounds
ie: NaCl
What are non electrolytes?
do not dissociate into ions when dissolved in water
do not conduct electricity
mostly molecular compounds
ie: C12H22O11
*not acids
Explain how sugar dissolves and how that differs from a sodium chloride solution?
sugar dissolves because the attraction between sugar and water molecules overcome the attraction of sugar molecules to each other. So, unlike a sodium chloride solution (which breaks up into Na+ and Cl- ions), a sugar solution contains intact C12H12O11 molecules homogeneously mixed with water molecules
What is a strong acid?
completely ionize in solution, strong electrolytes, single →
ie: HCl → H+ + Cl-
What is a weak acid?
do not completely ionize in water, weak electrolytes, use double arrow (⇌) in equations, eg: CH₃COOH ⇌ H⁺ + CH₃COO⁻
What is solubility?
grams solute that can be dissolved in 100 g solvent (water) at a temperature in Celsius, to be said simpler: whether something dissolves or not
What does it mean if something is soluble?
dissolves in water
What does it mean if something is insoluble?
does not dissolve in water
How do you determine solubility?
look at the given paper and see the rules and exceptions
What are precipitation reactions?
a solid (precipitate) forms when two solutions mix
*only insoluble compounds form precipitates
*if both possible products are soluble, no reaction occurs and no precipitate forms
What do you do when no reaction occurs?
write “no reaction,” cross out the arrow, and do not write the products
How do you write a precipitate reaction?
write formulas of two compounds as reactants
write products that could form by combining cations and anions
use solubility rules to determine if products are insoluble (s)
if all possibly products are soluble, write no reaction; if soluble, write (aq), if insoluble, write (s)
balance
What is a molecular equation?
complete neutral formulas for each compound
In actual solutions of soluble ionic compounds, how are dissolved substances presented?
as free ions, not as intact compounds
What is the complete ionic equation?
all of ions present (reactants and products) and shows states for each
ie: shows all soluble ions separated, indicating their participation in the reaction.
strong electrolytes are represented as their component ions in ionic equations— weak electrolytes are not
*liquids and solids do not break up, only aqueous
Write an example complete ionic equation for the reaction between lead (II) nitrate and potassium chloride.
The complete ionic equation shows the dissociation of lead (II) nitrate and potassium chloride in aqueous solution, resulting in lead (II) ions, potassium ions, and chloride ions, while maintaining the solid state of lead (II) chloride. It can be represented as: Pb^{2+} (aq) + 2 K^{+} (aq) + 2 Cl^{-} (aq) → PbCl_{2} (s) + 2 K^{+} (aq).
What are spectator ions?
do not participate in the reaction (same on both sides of the arrow)
What is the net ionic equation?
only species that actually change during the reaction and exclude spectator ions.
Write the net ionic equation for the reaction between lead (II) nitrate and potassium chloride.
The net ionic equation is Pb^{2+} (aq) + 2 Cl^{-} (aq) → PbCl_{2} (s).
What is an acid-base reaction?
“neutralization reaction,” acid reacts with base and they neutralize each other, producing water (sometimes weak electrolyte)
What is a gas-evolution reaction?
gas forms, bubbling (many are acid-base reactions)
What is the Arrhenius definition of an acid?
produces H+ (or H3O+) in an aqueous solution
What is the Arrhenius definition of a base?
produces OH- in an aqueous solution
What are strong acids?
dissociate completely, single arrow
What are weak acids?
do not dissociate completely, double arrow
What are polyprotic acids?
have more than 1 ionizable proton, release them sequentially
ie: H2SO4 → H+ + HSO4-
HSO4- → H+ + SO42-
What happens when you mix acids and bases (weak or strong)?
H+ (aq) from the acid combines with OH- (aq) form the base to form H2O (l) and usually an ionic compound, called a salt
What are the six strong acids?
The six strong acids are
H2SO4, HI, HBr, HNO3, HCl, HClO4
They completely dissociate in water.
What is a way to remember the six strong acids?
One mnemonic is "So I Brought No Clean Clothes" which stands for: HCl, HBr, HI, HNO₃, HClO₄, and H₂SO₄.
Any acids that are not the six strong acid list are considered what?
weak acids