Ionisation energy trends
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
First ionisation energy
The energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions
Second ionisation energy
The energy required to remove one electron from each ion in one mole of gaseous 1+ ions to form one mole of gaseous 2+ ions.
Third ionisation energy
The energy required to remove one electron from each ion in one mole of gaseous 2+ ions to form one mole of gaseous 3+ ions
Factors affecting ionisation energy
1. Nuclear charge
2. Distance from nucleus
3. Shielding
1st ionisation energy of Mg
Mg ---> Mg+ and e-
Second ionisation energy of Mg
Mg+---> Mg2+ and 2e-
Why do 1st Ionisation energies (generally) increase across a period?
Increased number of protons = stronger nuclear attraction. Little extra shielding or distance to lessen attraction from nucleus
Explain the drop between group 2 and 3 across period 3 (Mg and Al)
>Al's outer electron = in a 3p orbital rather than a 3s (Mg). 3p orbital has higher energy than 3s so is mostly found further from the nucleus>3p orbital has extra shielding from 3s electrons>Both factors override the increased nuclear charge>Easier to remove outer electrons so lower IE
Explain the drop between groups 5 and 6 across period 3(P and S)
>Identical shielding>The first ionization energy decreases slightly between group 5 (Phosphorus - P) and group 6 (Sulfur - S) across Period 3 due to the pairing of electrons within the 3p orbitals for the first time. In Phosphorus, all 3p electrons are unpaired, making it a stable configuration. In Sulfur, the 4th 3p electron is paired, leading to increased electron-electron repulsion, which makes it easier to remove and thus decreases the ionization energy.
Do Ionisation Energies increase with succession or decrease?
Increase
Why do the 1st ionisation energies decrease down groups?
Successive elements down a group have Increased Shielding and Increased distance from nucleus, nucleus' attraction to valence electron will be reduced Easier to remove outer electrons = lower IE
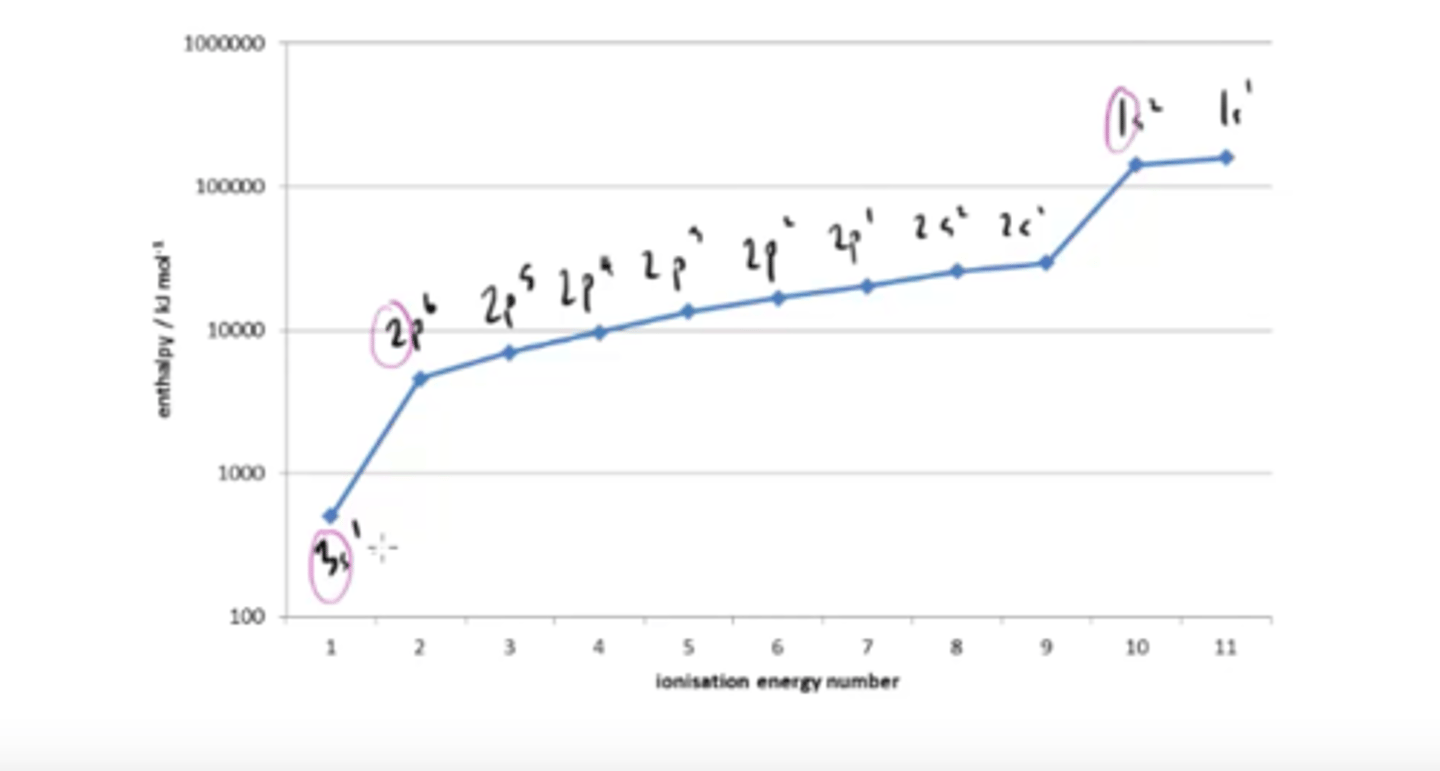
Why are there large jumps in successive ionisation energies
large jumps reveal where electrons are being removed from the next principal energy level.
effect of atomic radius on ionisation energy
As atomic radius increases, ionisation energy decreases
effect of nuclear charge on ionisation energy
ionisation energy increases as nuclear charge increases
effect of shielding on ionisation energy
the higher the shielding effect the lower the ionisation energy.
How can you work out which group of the periodic table an element belongs to from a successive ionisation energy graph?
Count how many electrons are removed before the first big jump
How can you predict the electronic structure of an element from a successive ionisation energy graph?
Working from RIGHT to LEFT count how many points there are before each big jump to find how many electrons are in each shell