naming hydrocarbons
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
cl
chloro
br
bromo
fl
fluro
iodo
CH3…
methyl
C2H5..
ethyl
C3H7
propyl
empirical form ethane
CH3
molecular form ethane
CH3CH3
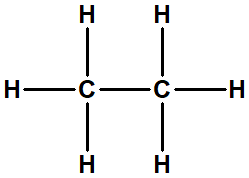
what representation
ethane: structural
condense formula ethane
CH3-CH3 H3C—-—CH3
what gets the lowest number in an alkene
double bond
always number the bond double or triple for alkanes and alkenes
yes
side changes are name as
akyl groups (methyl, ethyl)
structural isomers
when there are several carbons in the alkane and you start to connect the atoms in different ways
isomers
compounds within the same molecular formula but different structural formula
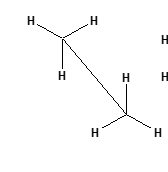
isomerism in alkanes: geometric isomer: staggered conformation
more stable, atoms are more spaced out
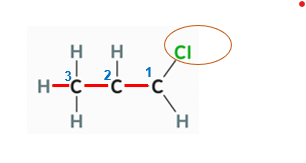
1-Chloro-propane
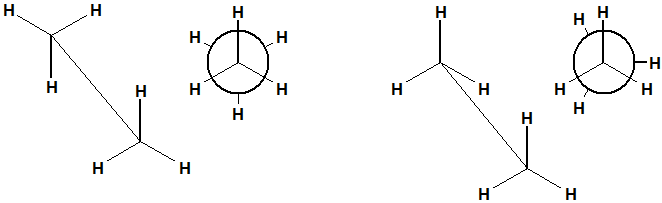
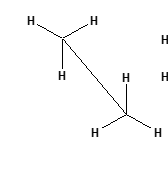
isomerism geometric isomers eclipsed
less stable, atoms overlap more- right one
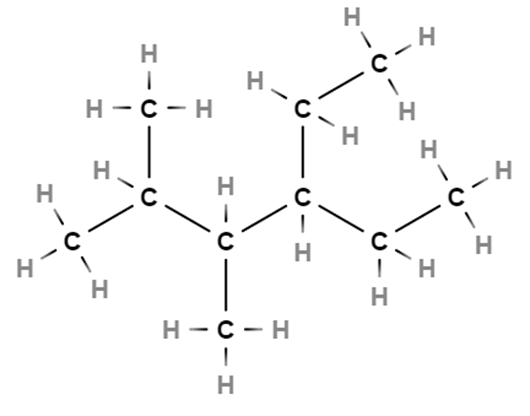
4-ethyl-2,3-dimethylhexane

isomerism in alkenes: structural isomers
structural isomers have different connectivity of atoms
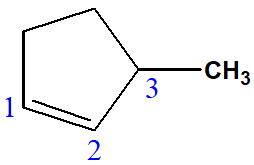
3-methylcyclopent-1-ene
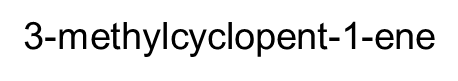
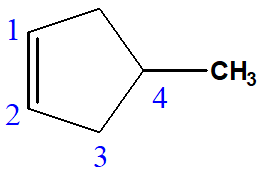
4-methylcyclopent-1-ene
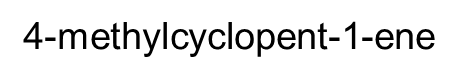
The presence of the double bond in alkenes adds another
factor to isomerism and to naming the substance
The location of the double bond is given to the
lowest carbon number in the chain
C=C bond cannot?..
rotate
The groups at the end of the restricted bond must be (isomerism in alkenes.
different
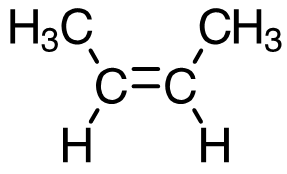
cis-but-2-ene
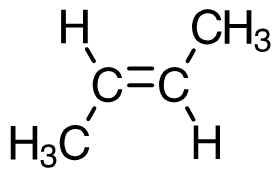
trans-but-2-ene
naming alkenes and alkynes: identify, number, suffix
identify the longest chain containing bond, number with multiple bond at lowest number, suffix ene or yne
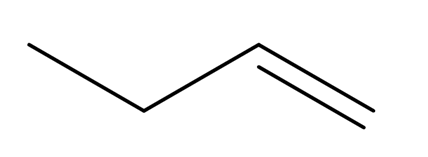
But-1-ene
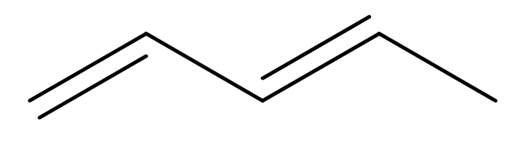
Pent-1,3-diene
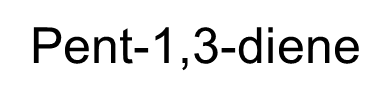
if more than one double/triple bond use
-diene or -triene and number positions of each bond
2,2-dichlorohex-3-ene.
cycloalkanes and cycloalkenes add__ and number

add cyclos before the root name and number the ring starting from the multiple bond or substituent
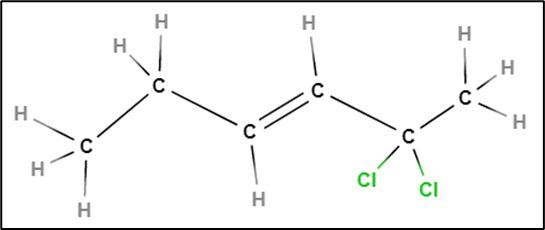
Cyclohexane

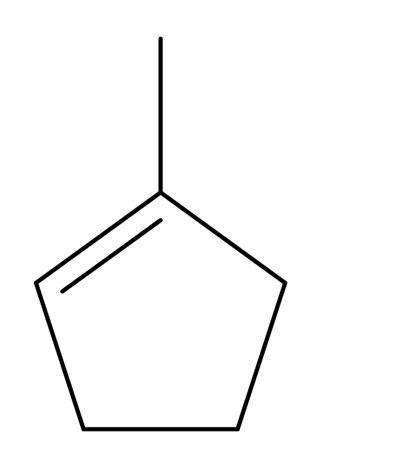
1-methylcyclopentene
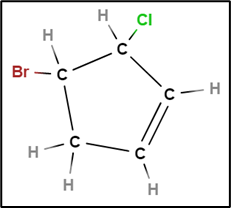
4-bromo-3-chlorocyclopent-1-ene or
4-bromo-3-chlorocyclopentene
aromatic compounds are named like a normal cyclic hydrocarbon however
the base name is benzene
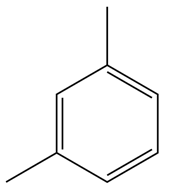
1,3-dimethylbenzene

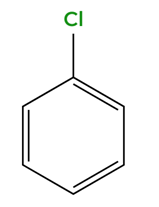
chlorobenzene
If two substituents in naming aromatic compounds use
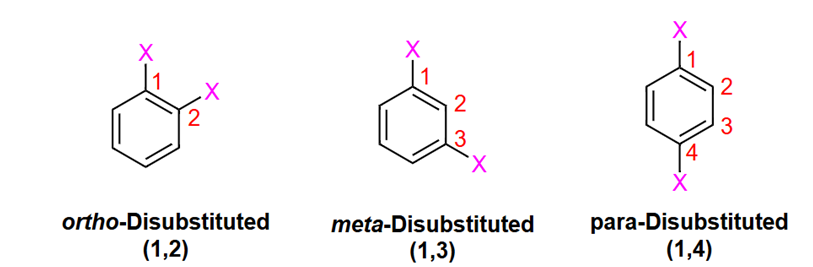
ortho (1,2) meta (1,3) para (1,4)
ortho, meta, para
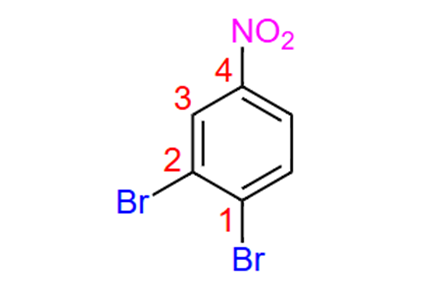
1,2-Dibromo-4-nitrobenzene
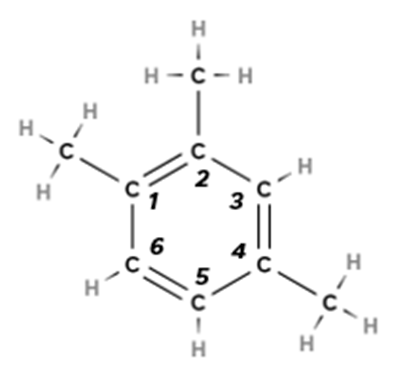
1,2,4-trimethylbenzene
non
9
dec
10
hept
7
prop
3
but
4
meth
1