Chemistry Intensified - Periodic Trends/Nuclear Reactions/Half Life
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
What is the equation for half life?
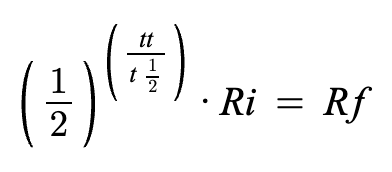
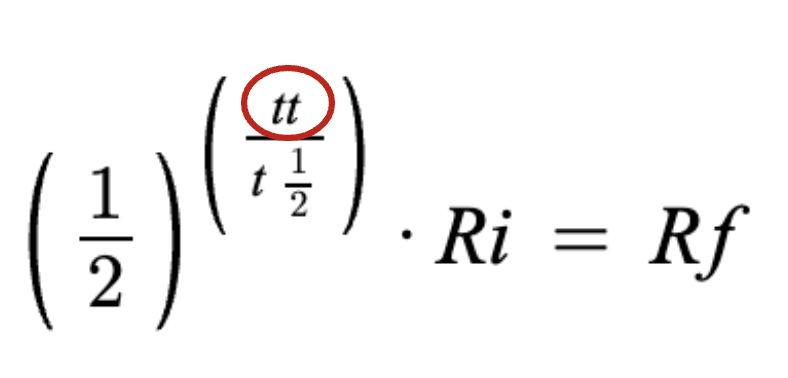
TOTAL time sample was around
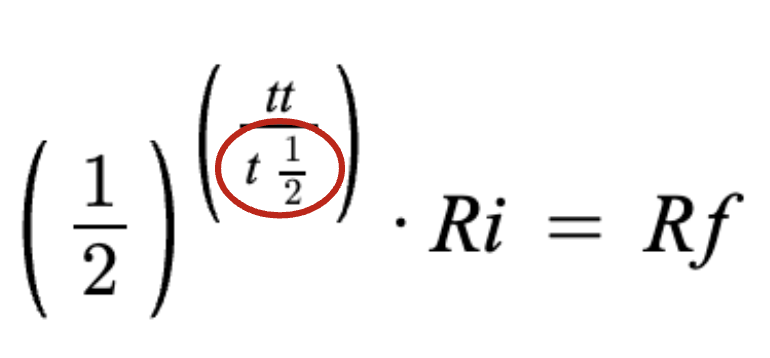
half life
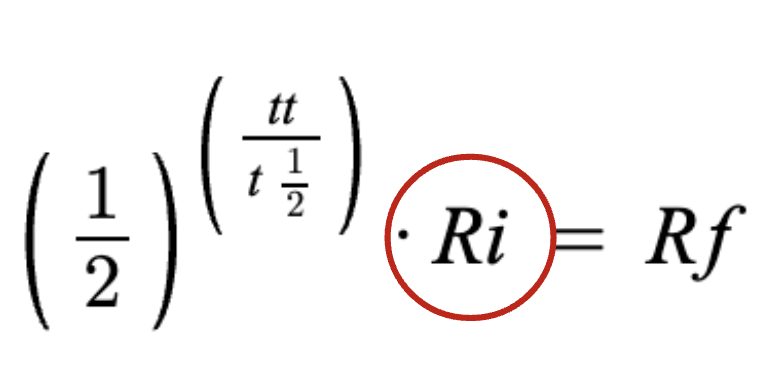
starting amount
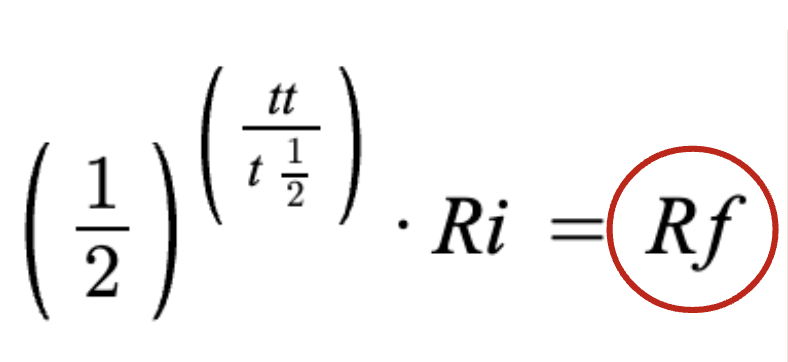
final amount
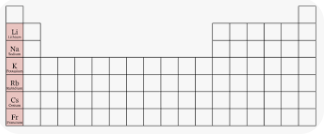
alkali metals
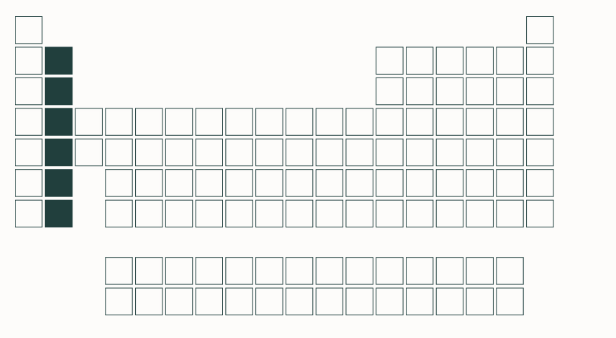
alkaline earth metals
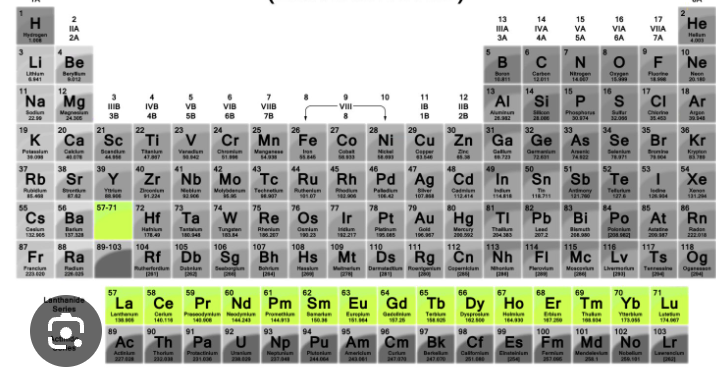
lanthanide
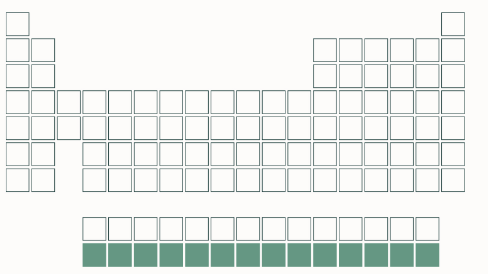
actinide
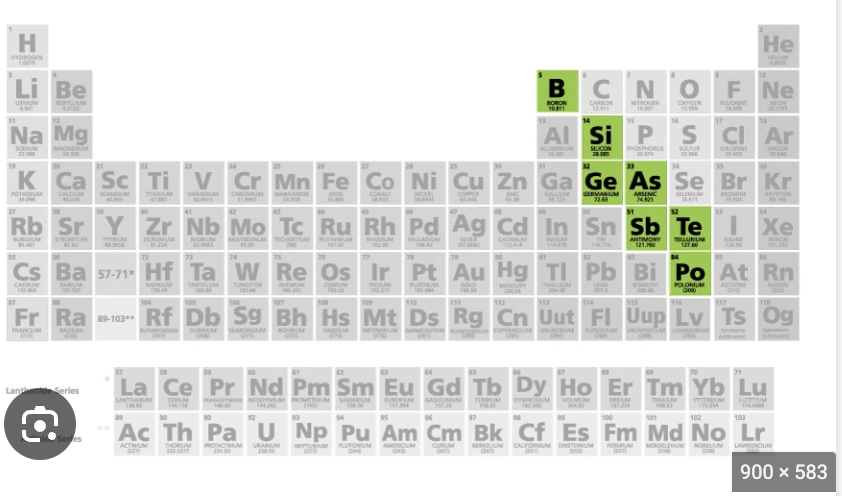
metalloids
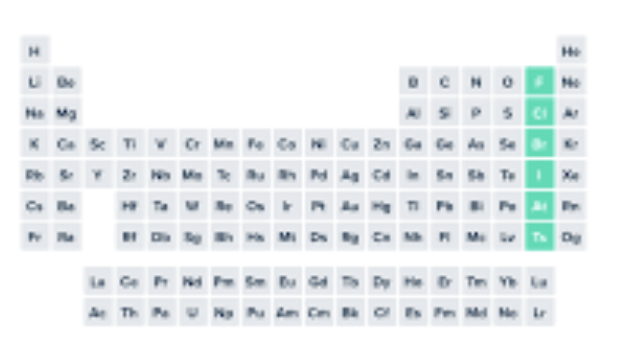
halogens
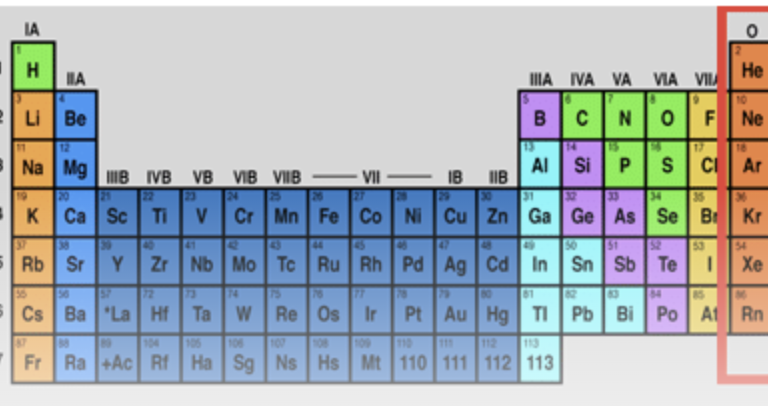
noble gases
What is coloumbs law?
the greater the distance, the less attraction there is
the less distance, the more attraction there is
What are valence electrons?
electrons in HIGHEST energy level
What are core electrons?
all electrons EXCEPT valence electrons
What is the effective nuclear charge?
STRENGTHENING of the nucleus across a period
What is atomic radius/TREND
distance measured from nucleus to outer electron trend
DECREASES as you go across the period
INCREASE as you go down the group
What is ionization energy/TREND
energy required to remove the last electron from an atom
INCREASE as you go across the period
DECREASE as you down the group
What is a cation?
an ion with a POSITIVE charge
What is an anion?
an ion with a NEGATIVE charge
What is ionic radius/TREND?
distance from nucleus of an ION to electron cloud
DECREASE as you across the period
INCREASE as you go down the group
Who is Johann Dobreiner
classified elements into TRIADS based on mass averages
Who is John Newlands
arranged elements into OCTAVES by increasing atomic mass
Who is Dimitri Mendeleev?
predicted properties of unknown elements, designed a table that had arrangement of elements based on atomic mass
Who is Henry Moseley?
developed moseleys law, showed relationship between atomic wavelength and number
How to determine if attraction force is stronger/weaker
as you go across a period, the attractive force increases
as you go down a group, it decreases
How to determine if valence electrons are closer/farther from nucleus
as you go down the group, ve distance increases
as you go across the period, ve distance decrease
shielding effect
effective nuclear charge
octave rule?