1.10 Solubility 💧
1/12
Earn XP
Description and Tags
GCSE CCEA Specification GCSE Chemistry Double Award Science, Triple Award Science Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Solubility
mass of solid that will saturate 100g of water (or solvent) at particular temperature
14g/100g water at 40°C
14g of solid is required to saturate 100g of water at 40°C
1cm3
= 1ml
1g
= 1000mg
1 litre
= 1000ml/ 1000cm3
Saturated solution
no more solute will dissolve at that temperature
Solubility curve
graph showing solubility of substance plotted against temperature
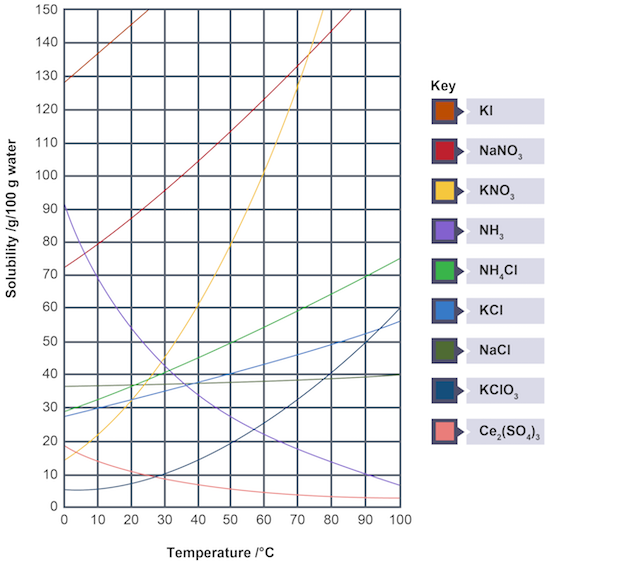
Temperature and solubility of solids
as temperature increases, solubility increases
Why crystallisation happens
solubility of salt/ solid decreases as solution cools
Hot, concentrated solution is cooled
some of the solute is deposited
Mass of solid deposited if solution containing 15g/100g of water at 30°C is cooled to 10°C
Use solubility curve to find at 10°C (10g)
Water can only dissolve 10g, not entire 15g
Mass of solid deposited = 15g - 10g = 5g
Temperature and solubility of gases
as temperature increases, solubility decreases
Different substances
have different solubilities