26.2 identifying aldehydes and ketones
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
what is used to detect the carbonyl functional group in ketones and aldehydes
2,4-dinitrophenylhydrazine / 2,4-DNP, brady’s reagant
in the presence of a carbonyl group, a yellow or orange precipitate called a 2,4-dinitrilehydrazone is produced
how to carry out the test for a carbonyl group in aldehydes and ketones
add 5cm depth of a solution of 2,4-dinitrophenylhydrazine to a clean test tube
using a dropping pipette, add three drop of the unknown compound
if no crystals for, add a few drops of sulphuric acid
a yellow/orange precipitate indicates the presence of an aldehyde or ketone
the impure yellow/ orange solid is filtered to remove the solid precipitate from the solution
the solid is then recrystallised to produce a pure sample of crystals
the melting point of the purified 2,4-dinitrophenylhydrazone is measured and recorded
how to tell the difference between an aldehyde or a ketone
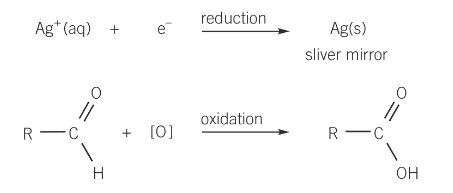
use tollens reagant which will produce a silver mirror if an aldehyde group is present
how to carry out the tollens test
pour 2cm³ of the unknown solution into a test tube
add an equal volume of tollens solution
leave the test tube to stand in warm water (about 50 degrees) for 10-15 minutes and observe whether any silver mirror is formed
the oxidation + reduction reactions that happen in the tollen’s test