material science 104
1/45
Earn XP
Description and Tags
quiz 1 material
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
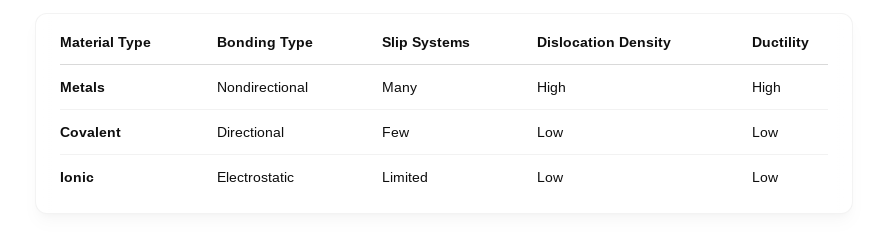
why are the number of dislocations present greatest in metals
non-directional bonding:
Metallic bonds involve a "sea of electrons" shared among many atoms, which means the bonds aren’t rigidly fixed in a particular direction. This allows atoms to slide past one another more easily, which helps dislocations form and move.
closely packed
Many metals have close-packed crystal structures like FCC (face-centered cubic) and HCP (hexagonal close-packed).
These structures have high atomic packing density and many slip systems — combinations of slip planes and directions where dislocations can move easily.
The ionic ones if you are trying to shear the positive ions will want to repel.
how are strength and dislocation motion related
stronger material slippage is harder, harder to deform
why does heating alter strength and other properties
how does plastic deformation often occur
in metals by slip ( an edge dislocation slides over adjacent plane half-planes of atoms
how to find plane where easiest slippage occurs
slip plane → highest planar densities
slip directions
direction of movement → highest linear densities
strategies for strengthening
reduce grain size: grain boundaries are barriers to slip, smaller grain size more barriers, the strength of barriers increase with increasing angle of misorientation
form solid solutions: (mixture of atoms in solid, impurity atoms distort the original lattice and generate lattice strains which act as barriers to dislocation motion) (small or big impurity)
precipitation strengthening: space smaller yield strength higher. forming tiny particles (precipitates) inside the metal’s crystal structure. tiny precipitates block the motion of dislocations, making plastic deformation harder
cold work: forging, drawing (pull metal rod through narrow passage of dies) rolling, extrusion. All happen at room temperature.
dislocation density
total dislocation length / Unit volume (increases during cold working)
yield strength
strength before material plastic-ally deforms (0.002)
impact of cold working
increase yield strength, tensile strength but ductility decreases
annealing stages (heat treating after cold working)
recovery: reduction of dislocation density by annihilation
re-crystallization: more homogeneous/more uniform they consume and replace parent cold work grains until consumed
grain growth
recrystallization temperature
temperature at which recrystallization reaches completion in 1hr (Tr decreases with increasing %CW, decreases with increasing purity)
relationship between properties, structure, processing
Properties depend on structure
Processing can change structure
material selection process
Pick APPLICATION → determine required properties (mechanical, electrical, thermal, magnetic, optical, deteriorative)
Properties → identify candidate materials (materials: structure, composition)
material → identify required processing (processing: changes structure and overall shape
types of materials (metals, polymers, ceramics)
metals
strong, ductile
opaque, reflective
high thermal & electrical conductivity
metallic bonding
polymers
covalent bonding (sharing of electrons)
thermal & electrical insulators
optically translucent or transparent
ceramics
ionic bonding
compounds of metallic and non metallic elements
non-conducting (insulator)
brittle, glassing, eleastic
primary bonds
what promotes bonding ?
what types of bonds are there
what properties are inferred from bonding
why are most electron configurations not stable
because valence shell usually not filled completely
ionic bonds
metal (donates electrons) + nonmetal (accepts electrons)
electrostatic attractions between oppositely charged ions
large bond energy
nondirectional
covalent bonding
similar electronegativity (share electrons)
variable bond energy
directional
% ionic character MgO Xmg = 1.3 Xo =3.5
secondary bonding
arises from interactions between dipoles
fluctuating dipoles
permanent dipoles
small bond energy
properties from bonding: Tm (melting temperature) relation to bond energy (Eo)
Melting temperature is larger with larger bond energy which is dependent on bond length
properties from bonding: coefficient of thermal expansion
coefficient of thermal expansion is larger if bond energy is smaller. This value is the coefficient multiplied by heated temperature minus unheated temperature which is equal to change in length / initial length
polymorphism
some materials have more that one crystal structure
X-ray diffraction
used for crystal structure and interplanar spacing determinations
diffusion is faster for (structure, bonding, size of atoms, density)
open crystal structures
materials w/ secondary bonding
smaller diffusing atoms
lower density materials
how does diffusion occur
mass transport by atomic motion
interdiffusion
in an alloy, atoms tend to migrate from regions of high concentration to regions of low concentration
self-diffusion
in an elemental solid, atoms also migrate
vacancy diffusion
atoms exchange with vacancies
applies to substitutions impurities atoms
rate number depends on number of vacancies and activation energy to exchange
why is diffusion an important part of processing
how can the rate of diffusion be predicted for some simple cases
how does diffusion depend on structure and temperature
interdiffusion