Chapter 8: The Gas Phase (9%)
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
fluids, compressible
Gases are ______ and therefore conform to the shapes of their containers
They are also easily _____________
temperature, pressure, volume, number of moles (T, P, V, n)
the 4 variables that describe gas systems
increases, decreases
A simple mercury barometer measures incident (usually atmospheric) pressure.
As pressure ___________, more mercury is forced into the column, increasing its height.
As pressure ____________, mercury flows out of the column under its own weight, decreasing its height.
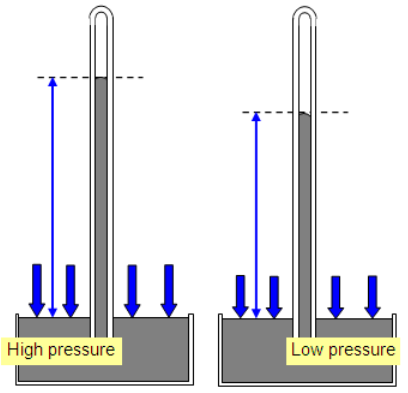
237K, 1atm
State the TEMPERATURE and PRESSURE defined as “standard temperature and pressure” (STP)
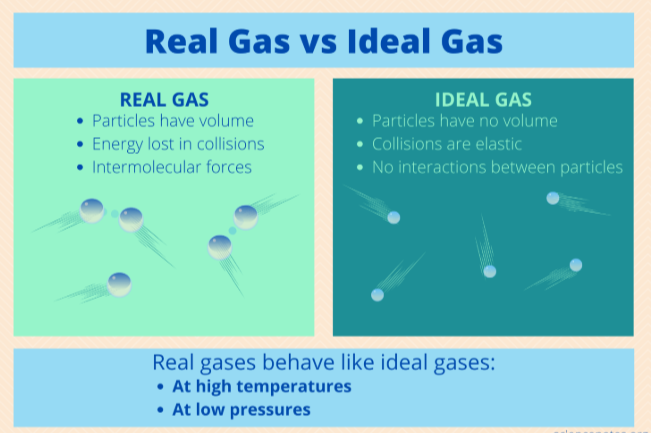
have no intermolecular forces, occupy no volume
the 2 characteristics of an ideal gas
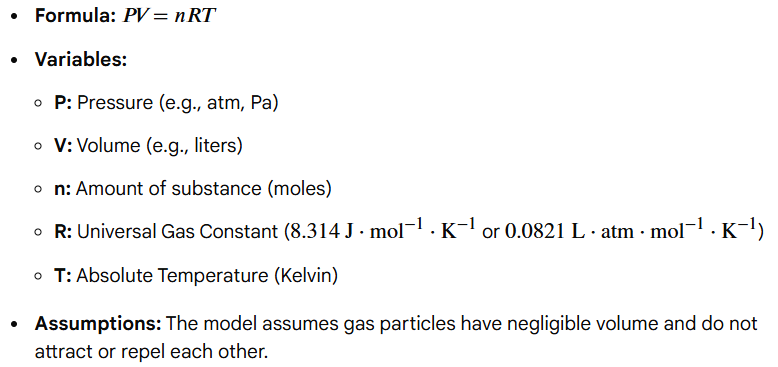
pressure x volume = number of moles x ideal gas constant x temperature
(PV = nRT)

Write out the formula for the Ideal Gas Law
Variables = pressure, volume, number of moles, ideal gas constant, temperature
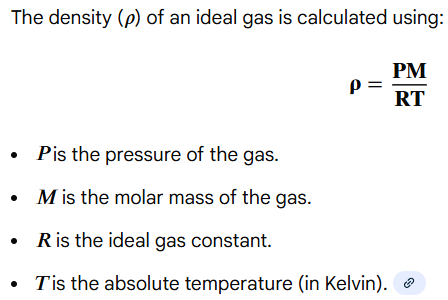
density = mass / volume = (pressure x molar mass) / (ideal gas constant x temperature)

Write out the formula for calculating the DENSITY of a gas (derived from the ideal gas law PV = nRT)
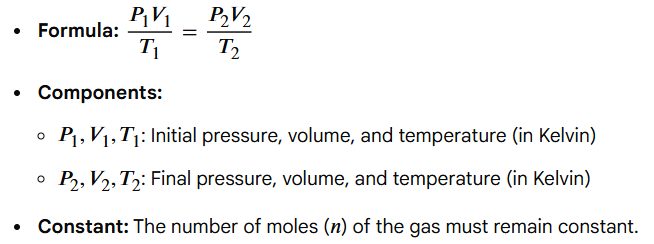
(P1V1)/T1 = (P2V2)/T2
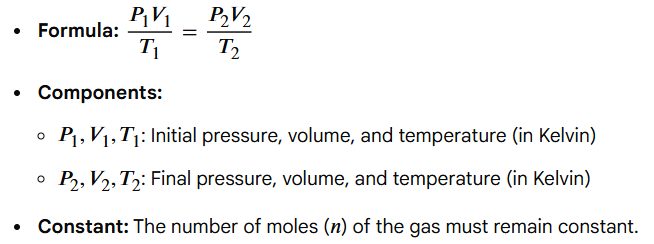
Write out the formula for the Combined Gas Law (derived from the ideal gas law PV = nRT)
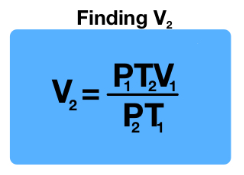
V2 = V1 (P1/P2) (T2/T1)

Write out the formula for calculating the CHANGE IN VOLUME (derived from the combined gas law P1V1/T1 = P2V2/T2)
volume
Regardless of the identity of the gas, equimolar amounts of two gases will occupy the same _________ at the same temperature and pressure
22.4 L
The volume that one mole of an ideal gas occupies at STP
pressure, P, temperature, T, direct, increases
Avogadro’s Principle is a special case of the Ideal Gas Law (PV = nRT) for which the __________ (___) and ______________ (___) are held constant
It shows a _________ relationship between # of moles of gas (n) and volume (V), where increasing one ___________ the other


V1/n1 = V2/n2

Write out the formula for Avogadro’s Principle in which pressure (P) and temperature (T) remain constant
temperature, T, number of moles, n, inverse, decreases
Boyle’s Law is a special case of the Ideal Gas Law (PV = nRT) for which the _____________ (___) and ________ ___ ______ (___) are held constant
It shows an _________ relationship between pressure (P) and volume (V), where increasing one ___________ the other
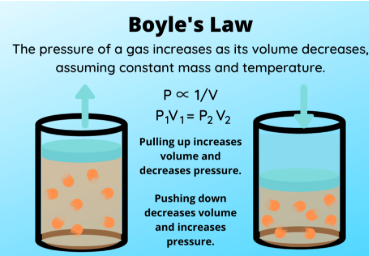
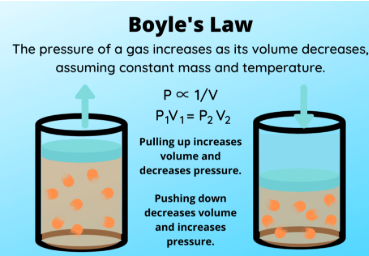
P1V1 = P2V2

Write out the formula for Boyle’s Law in which temperature (T) and number of moles (n) remain constant
pressure, P, number of moles, n, direct, increases
Charles’s Law is a special case of the Ideal Gas Law (PV = nRT) for which the __________ (___) and ________ ___ ______ (___) are held constant
It shows a _________ relationship between temperature and volume, where increasing one ___________ the other
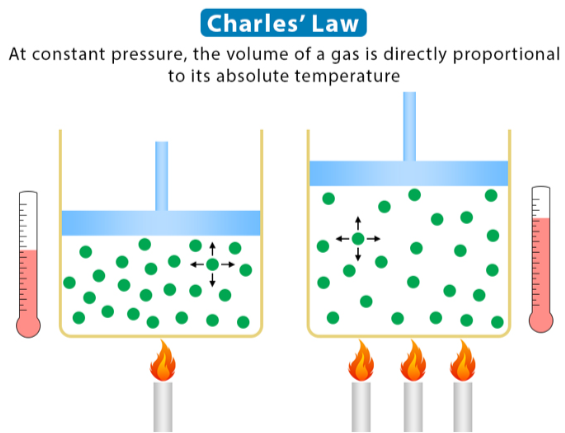
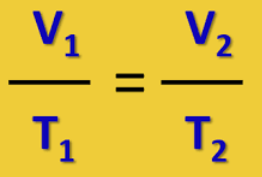
V1/T1 = V2/T2
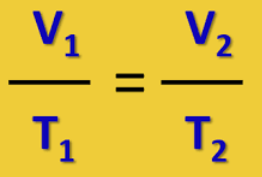
Write out the formula for Charles’s Law in which pressure (P) and number of moles (n) are constant
volume, V, number of moles, n, direct, increases
Gay-Lussac’s Law is a special case of the Ideal Gas Law (PV = nRT) for which the _________ (___) and ________ ___ ______ (___) are held constant
It shows a _________ relationship between temperature (T) and pressure (P), where increasing one ___________ the other
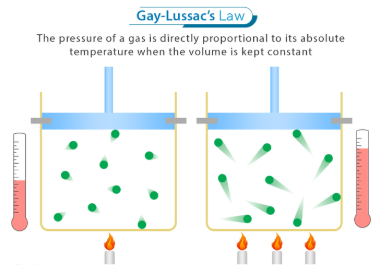
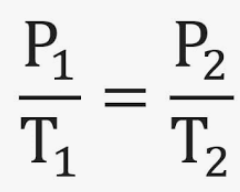
P1/T1 = P2/T2
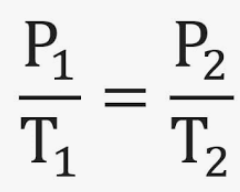
Write out the formula for Gay-Lussac’s Law in which volume (V) and number of moles (n) remain constant
direct, inverse
The Combined Gas Law shows a _________ relationship between pressure (P) and volume (V) along with _________ relationships between both pressure (P) and volume (V) with temperature (T)
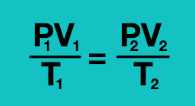
individual, pressures, mole fractions, pressure, partial pressures
Dalton’s Law of Partial Pressures states that ____________ gas components of a mixture of gases will exert individual __________ in proportion to their ______ _________.
The total _________ of a mixture of gases is = to the sum of the ________ _________ of the component gases.
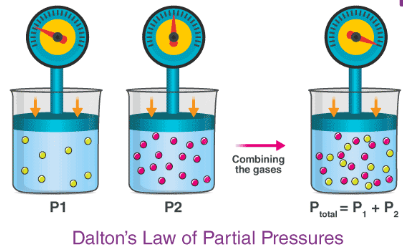
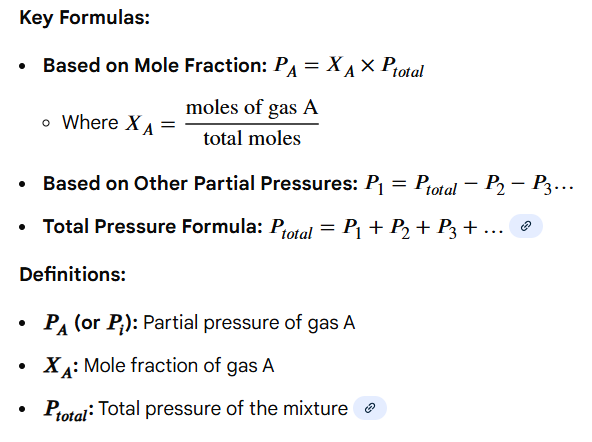
(moles of individual gas / total moles of gas) x total pressure

Write out the formula for calculating the PARTIAL PRESSURE of a gas according to Dalton’s Law of Partial Pressures
Variables: moles of individual gas, total moles of gas, total pressure
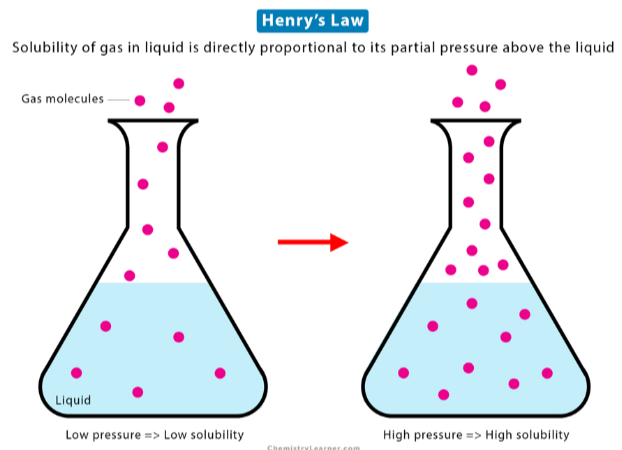
dissolved, partial pressure, vapor pressure
Henry’s Law states that the amount of gas ___________ in solution is directly proportional to the _________ __________ of that gas at the surface of the solution (aka its ________ __________)
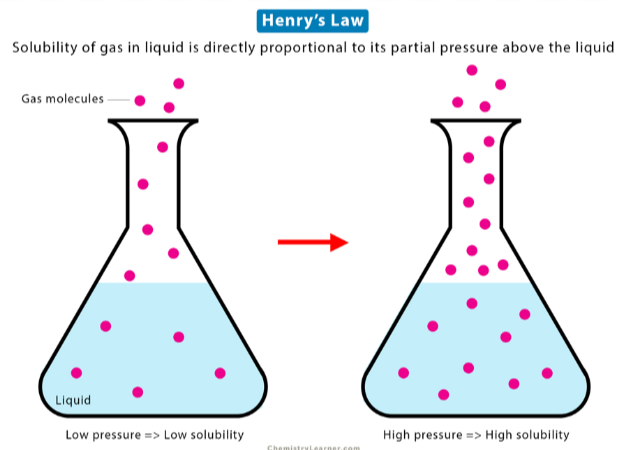
vapor pressure
The pressure exerted by evaporated particles above the surface of a liquid
increase
The solubility of a gas will __________ with increasing partial pressure of the gas
volume, intermolecular, random collisions, elastic, temperature
Kinetic Molecular Theory
According to the Kinetic Molecular Theory, which attempts to explain the behavior of gas particles, gas particles have negligible __________, they do not experience _____________ forces, they undergo __________ ___________ with each other and the walls of the container, collisions are ________, and the average kinetic energy of the gas particles is directly proportional to ______________
faster, slower
Kinetic Molecular Theory
The HIGHER the temperature, the _________ the molecules move.
The LARGER the molecules, the _________ they move.
faster
Graham’s Law states that gases with LOWER molar masses will diffuse or effuse ________ than gases with HIGHER molar masses at the same temperature
![<p>square root [molar mass of gas 2 / molar mass of gas 1]</p>](https://knowt-user-attachments.s3.amazonaws.com/a8b9a171-cc92-4019-aec8-3faeab1a68d5.png)
square root [molar mass of gas 2 / molar mass of gas 1]
Write out the formula for Graham’s Law which calculates the rates of diffusion for two gasses (diffusion rate of gas 1 / diffusion rate of gas 2 = ?)
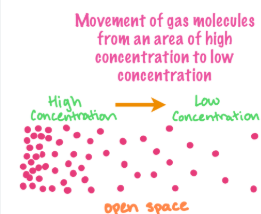
diffusion
Diffusion vs. Effusion
The spreading out of particles from high to low concentration
effusion
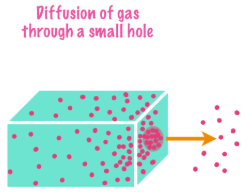
Diffusion vs. Effusion
The movement of gas from one compartment to another through a small opening under pressure
slower, slower
Heavier gasses dissolve _________ than lighter ones because particles with greater mass travel at a _________ average speed
low temperature, high pressure (aka low volume)
the 2 conditions under which real gases start to deviate from ideal behavior
less, intermolecular attractions
At moderately high pressures, low volumes, or low temperatures, real gases will occupy _____ volume than predicted by the ideal gas law because the particles have _____________ ___________
more, take up space
At extremely high pressures, low volumes, or low temperatures, real gases will occupy _____ volume than predicted by the ideal gas law because the particles ______ ___ _______