Stoichiometry and General Concepts
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What is a Mole?
It is an SI unit used to measure the amount of any substance.
It is equal to Avogadro’s # 6.022×1023
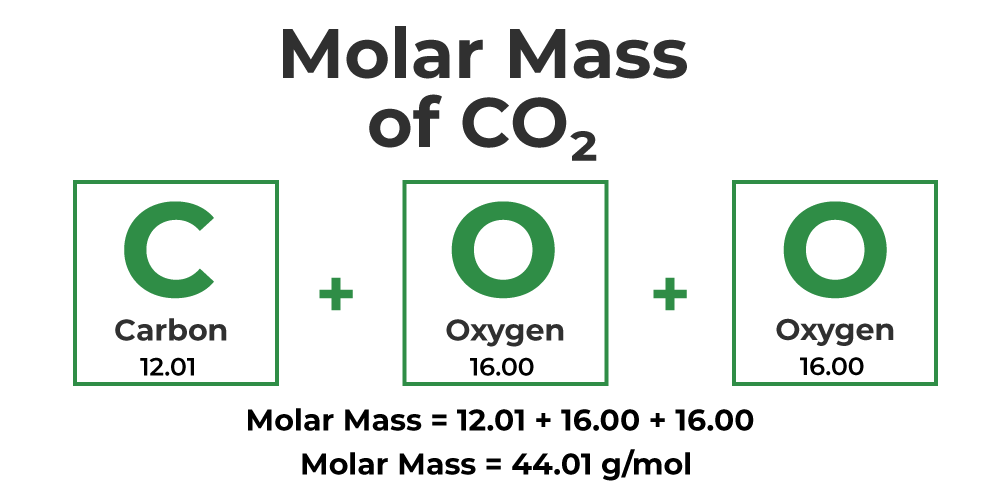
What is molar mass?
It is the sum of the mass of all atoms found in one mole of a substance.
It is expressed in units of grams per mole
How do you calculate mass to moles?
n (# of moles)= m (mass in grams)/ M(molar mass)
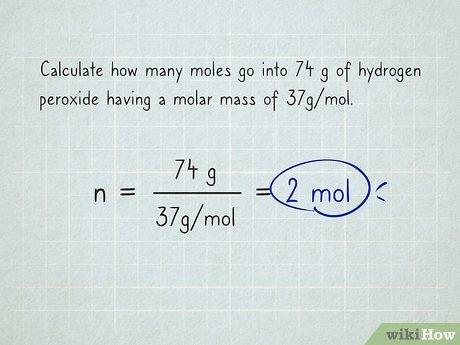
What is a mole ratio? ( mole to mole ratio)
A mole ratio is the ratio between the amounts of moles of any two molecules involved in a chemical reaction.
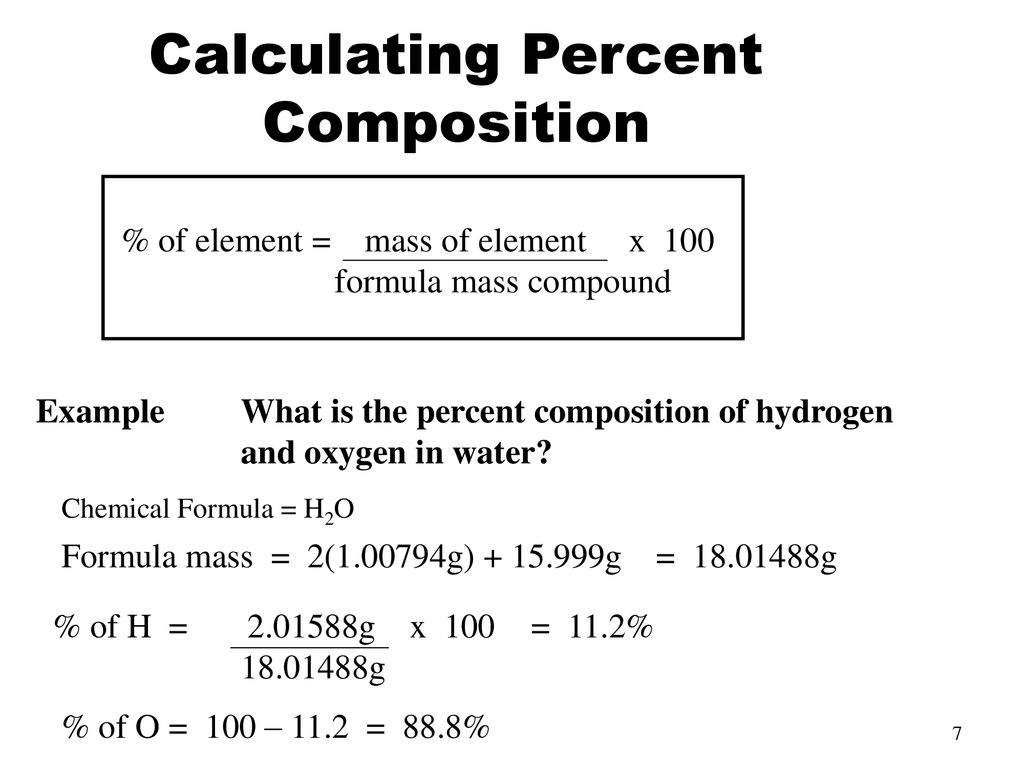
What is the formula for percent composition?
look at photo
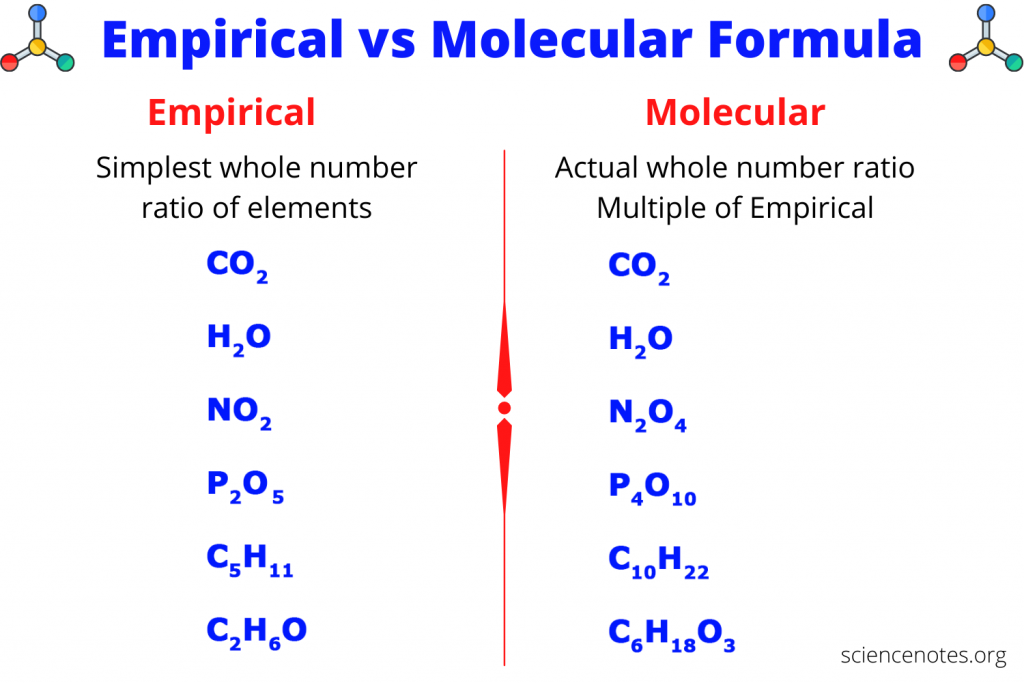
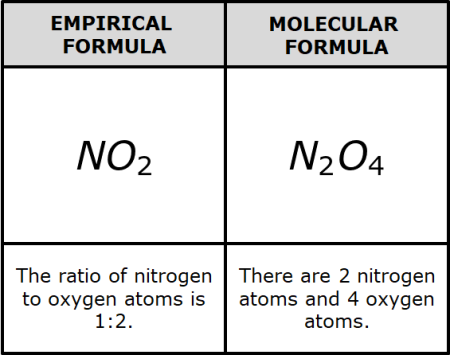
What is the molecular and empirical formula?
The molecular formula is considered the regular formula, and it is the number of atoms of each element in a compound.
The empirical formula is considered the reduced formula, and it is the simplest or most reduced ratio of atoms in a compound
How do you determine the empirical formula from a molecular formula?
Divide the molecular formula by the subscripts largest common factor to find the empirical formula
How do you determine the molecular formula from the empirical formula?
Calculate the molar mass of the empirical formula
Divide the given molecular molar mass by the molar mass calculated for the empirical formula
Multiply each subscript by the whole number that resulted from step two. This is now the molecular formula.
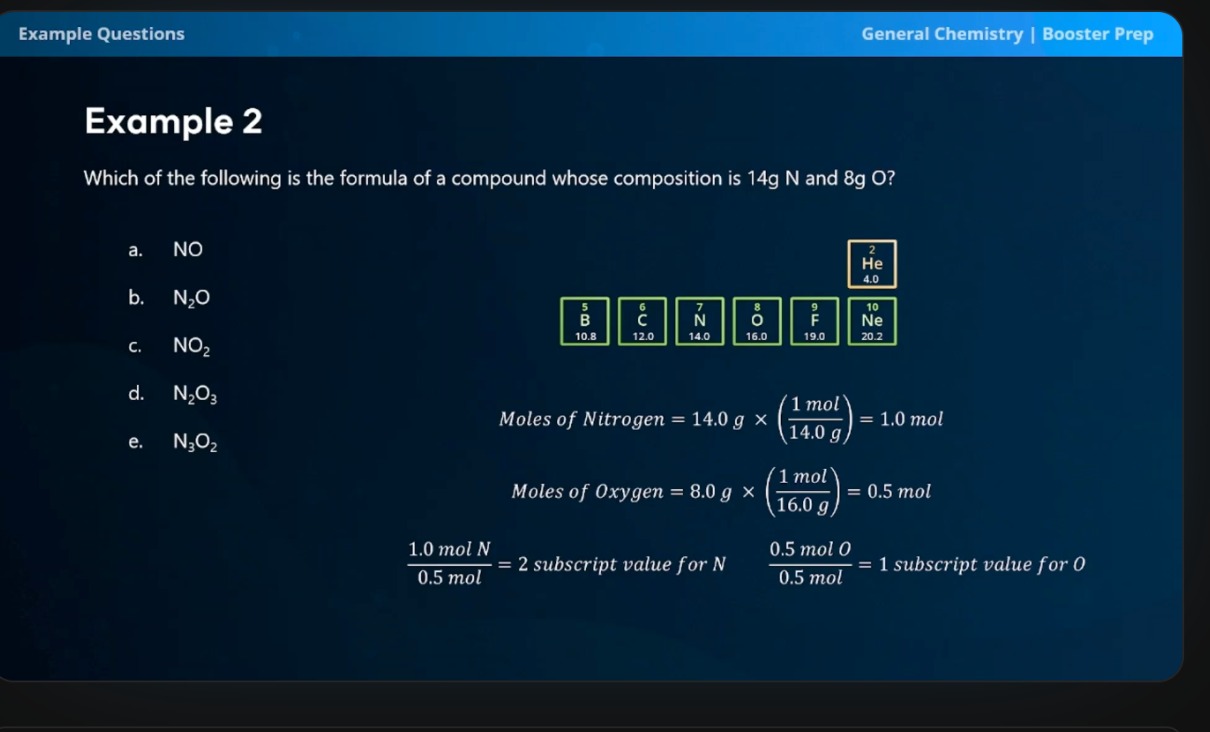
How do you determine the empirical formula from percent composition?
Assume 100 grams of total sample, converting percentage into grams
Divide each number of grams by the element’s atomic mass
Divide the number from step two by whichever is smaller
If necessary, multiply all subscripts by an integer to ensure that the smallest whole-number ratio is obtained.

What is a coefficient?
The # of each molecule that is used to balance the chemical equation (can be changed)
What is a subscript?
The # of atoms of an element in each molecule ( cannot be changed)
What is a limiting reagent?
The limiting reagent is the reactant that determines the amount of product formed. It is completely used up in a reaction.
How do you determine the limiting reagent?
Make sure the chemical equation is balanced. If not, balance the equation
Convert all the given information to moles
Pick one of the reactants as the limiting reactant and determine how much of the other reactant is required for the reactant that you chose
If there is an excess of the other reactant, the reactant you chose is the limiting reagent. If there is a shortage of one of the reactants, that reactant is the limiting reagent.
