Unit 9 - Thermodynamics and Electrochemistry
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
The entropy of an isolated system will always ____ (Second Law of Thermodynamics)
increase over time
when is enthalpy and entropy favored? (decreasing/increasing)
enthalpy favored when decreasing… (exothermic reactions favored)
entropy favored when increasing…
if both ΔH enthalpy (-) and ΔS entropy (+) are favored then what is true about temperature?
then the reaction will be favored (spontaneous) at all temps
if both ΔH enthalpy (+) and ΔS entropy (-) are UNFAVORABLE then what is true about temperature?
it will NEVER be favored (spontaneous) at any temp!!
If ΔH and ΔS are both +, the reaction is only (spontaneous) favored at..
high temps!
If ΔH and ΔS are both - the reaction is only (spontaneous) favored at …
low temps!
What is Gibbs Free Energy?
a measure of the thermodynamic favorability of a reaction
ΔG = ΔH - T(ΔS)
OR
ΔG = products - reactants
Ag2CrO4 has a ΔG=+68 kJ/mol is it soluble/insoluble?
insoluble because ΔG unfavorable (positive) so reaction to dissolve it wouldn’t be driven (atleast naturally..)
if its spontaneous in one direction it is ____ in the other
nonspontaneous
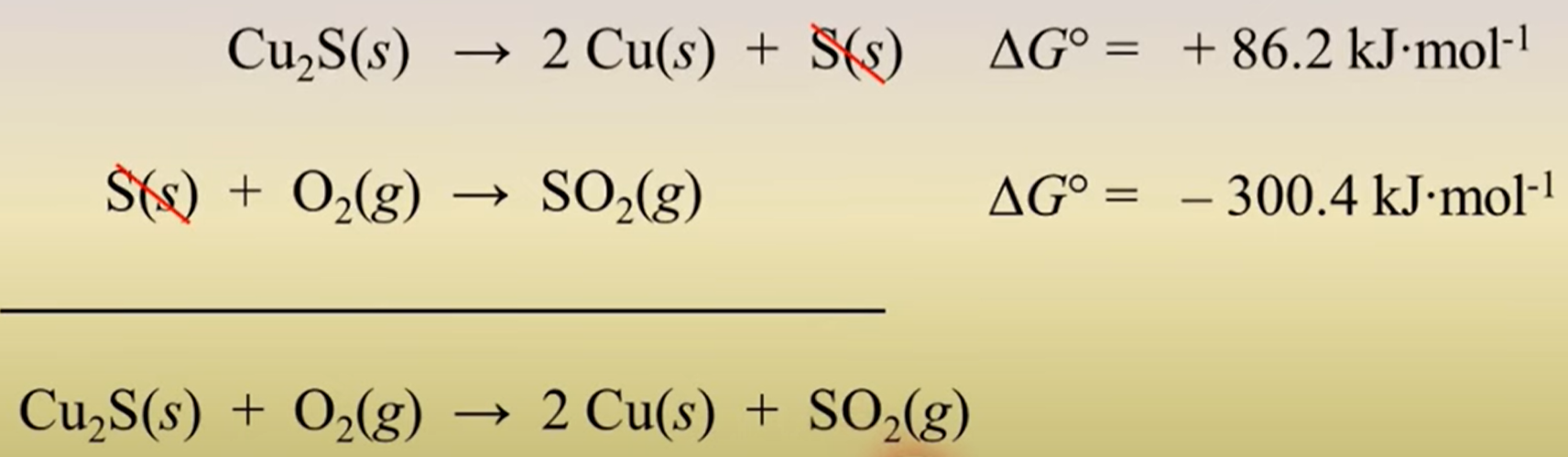
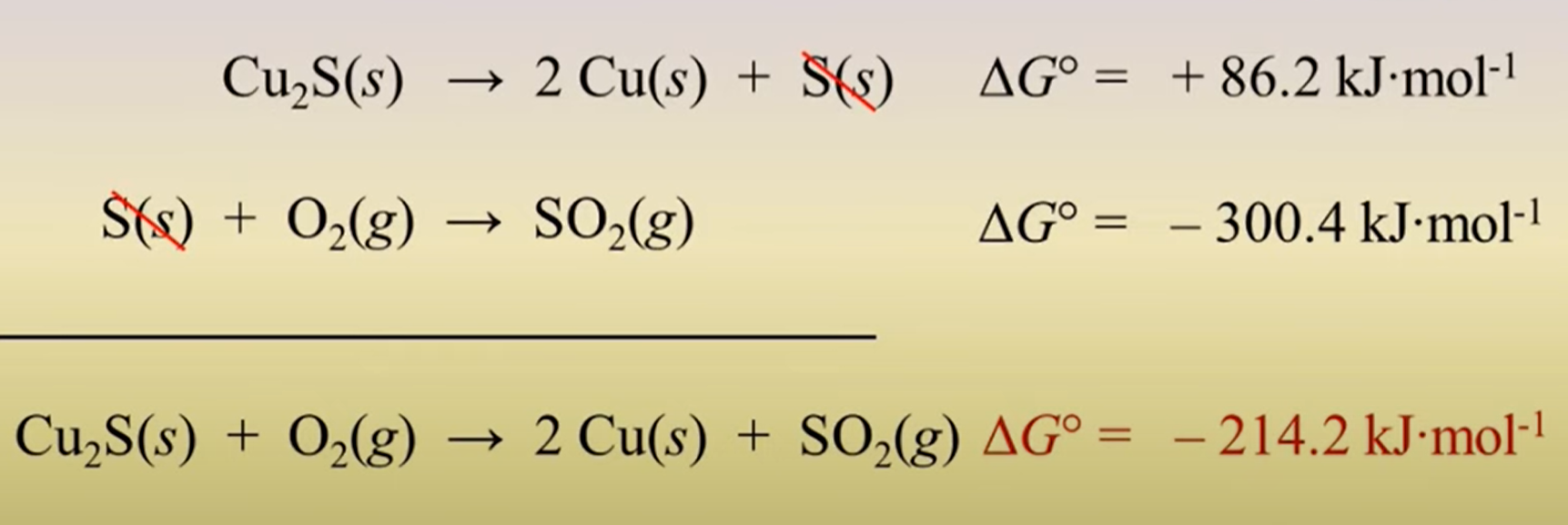
when combining two reactions what do you do with their ΔG? (add/multiple/subtract..)
add their ΔGs!
in galvanic cell anode ____ and cathode ___ in mass
anode decreases and cathode decreases in mass (cat gets fat..)
purpose of salt bridge
transfer of ions to keep charges all balanced
flow of ions in salt bridge (where do cations and anions go?)
anions towards anode
cations toward cathode
the ions in the salt bridge need to be ___
inert or nonreacting with the ions in the solutions
reduction takes place in the ___
cathode (red cat)
oxidation takes place in the ___
anode (an ox)
Galvanic cells are always…
thermodynamically favorable (spontaneous)
Which one is the anode and cathode from these reduction half reactions?
Fe2+ + 2e- → Fe -0.44V
Cu2+ + 2e- → Cu +0.34V
use Ecell = Ecathode - Eanode
and plug in either V value for either cathode or anode until you get a postive answer
Fe as cathode, Cu as anode : (-0.44V) - (0.34V) = -0.78 NOPE!
Cu as cathode, Fe as anode : (0.34V) - (-0.44V) = +0.78V YES!
If E°cell has that little degree symbol it means…
it’s at standard conditions
What are standard conditions?
Temp= 25C
Concentration(aq) = 1.00M
Pressure of all gases = 1.00 atm
In a galvanic cell, Ecell is thermodynamically favorable when it is positive/negative
positive E cell
Lets say Ecell is negative how would we drive the reaction forward?
with an electrolytic cell!! since -Ecell is unfavorable and wouldnt work in galvanic cell