Chemistry - Unit 3: Electronic Structure
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
metals
left of the staircase
usually solid
high melting point
shiny
malleable
good conductor
ready to lose electrons
nonmetals
right of the staircase
usually gas
low melting point
dull
brittle
poor conductor
ready to gain electrons
metalloids
on the staircase
moderate melting point
shiny or dull
malleable
medium conductor
lose/gains electrons
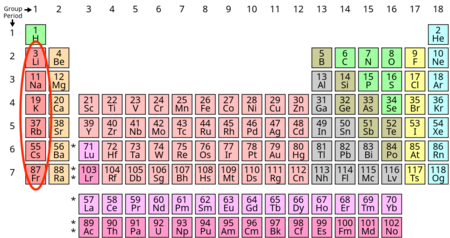
alkali metals
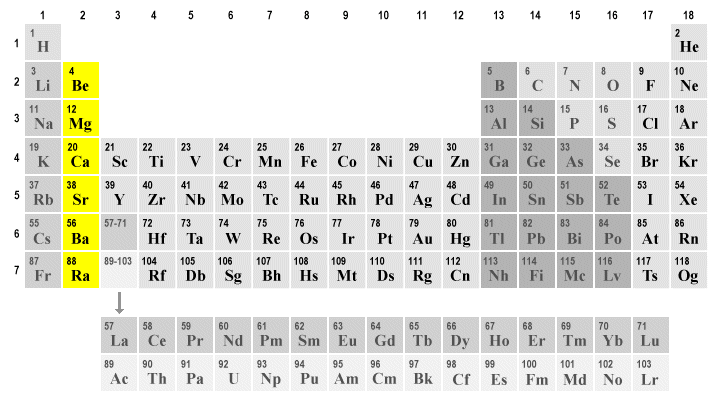
alkaline earth metals
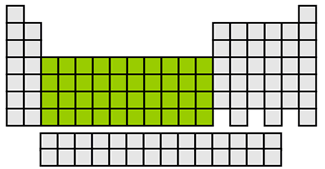
transition metals
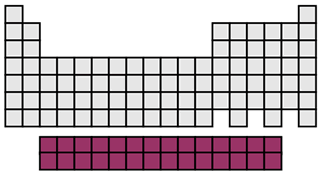
inner transition metals
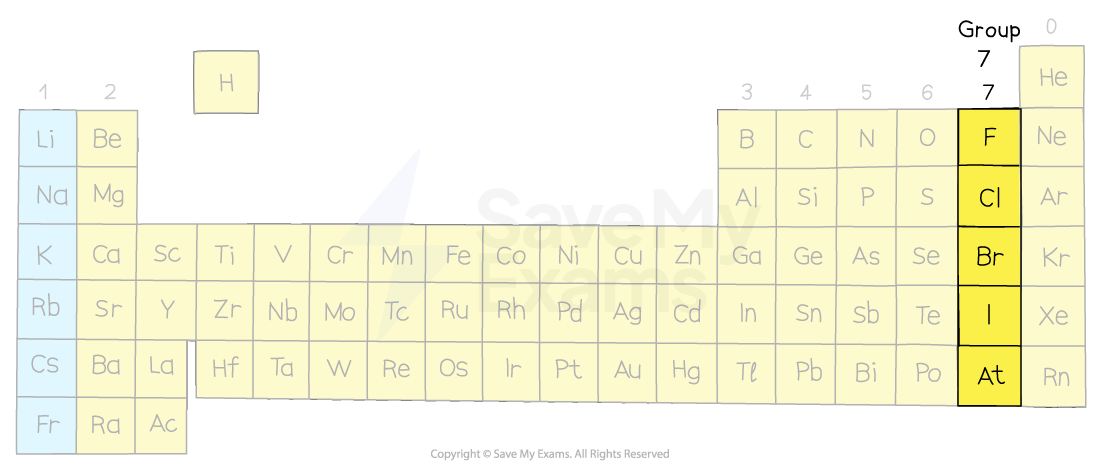
halogens
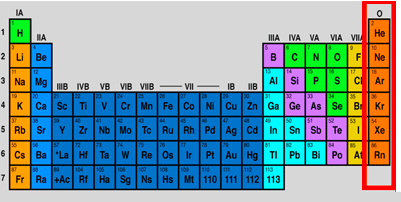
noble gases
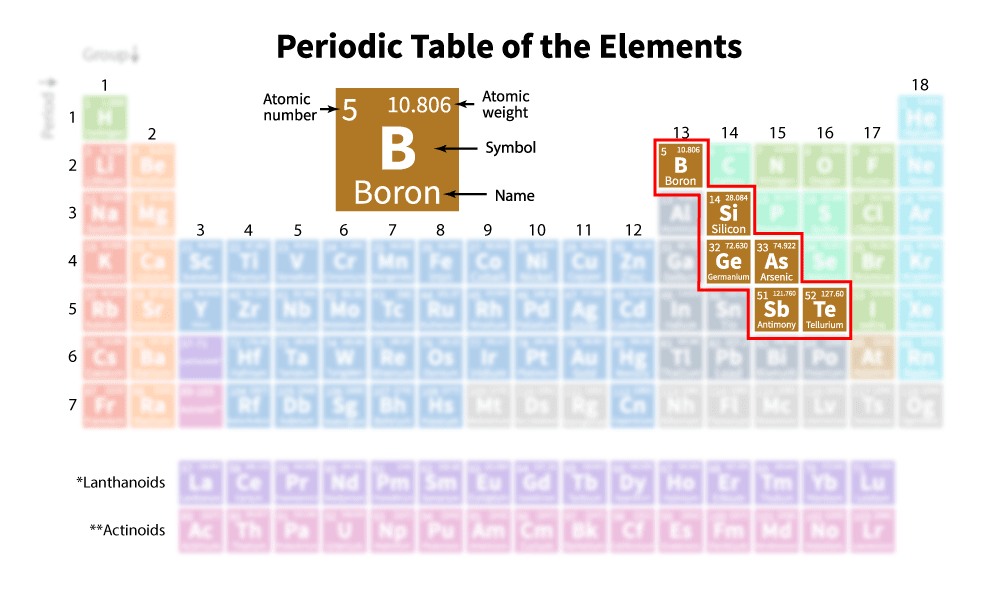
metalloids
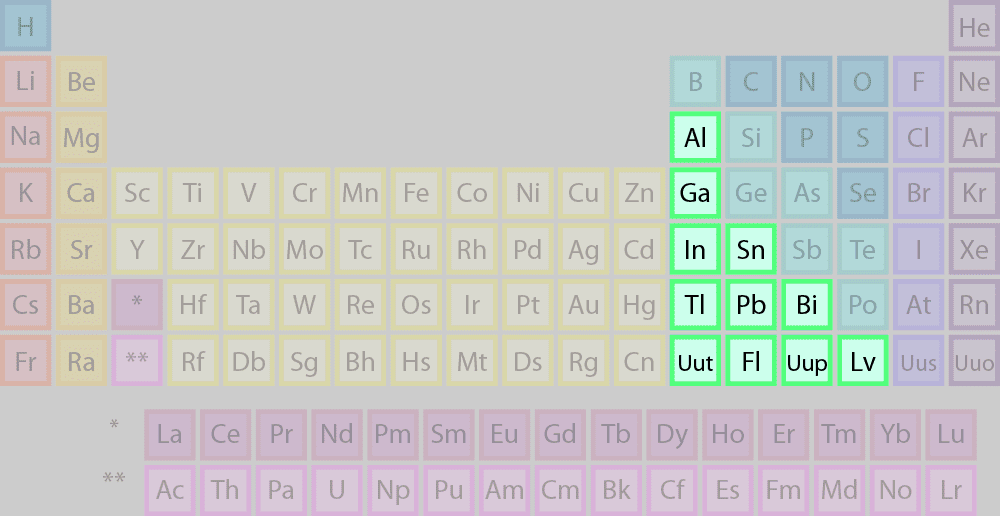
other metals
other non-metals
atomic size _____ as you move down a group
increases
atomic size ______ as you move across a period
decreases
ionization energy _____ as you move down a group
decreases
ionization energy ______ as you move across a period
increases
electronegativity ______ as you move down a group
decreases
electronegativity _____ as you move across a period
increases
alkali metals
1 valance e-
very high reactivity
soft metals
group 1
alkaline earth metals
2 valence e-
high reactivity
soft metals (harder than group 1)
group 2
transition metals
valence e- varies
moderate reactivity
hard metals
good heat/electrical conductors
groups 3-12(d block)
inner transition metals
valence e- varies
moderate reactivity
behave like alkali metals
all radioactive
halogens
7 valence e-
moderate reactivity
often form salts with metals
group 17
noble gas
8 valence e-
low-hone reactivity
mendeleev
arranged elements in order of increasing atomic mass
moseley
realized elements should be arranged by increasing atomic number
energy level
distance of the orbital from the nucleus
aufbau principle
states an electron will occupy the lowest energy orbital
schrodinger
determined the probability of finding an electron in various locations around the nucleus
electronegativity
ability to attract electrons when it a part of a compound
ionization
amount of energy required to remove e- from an atom